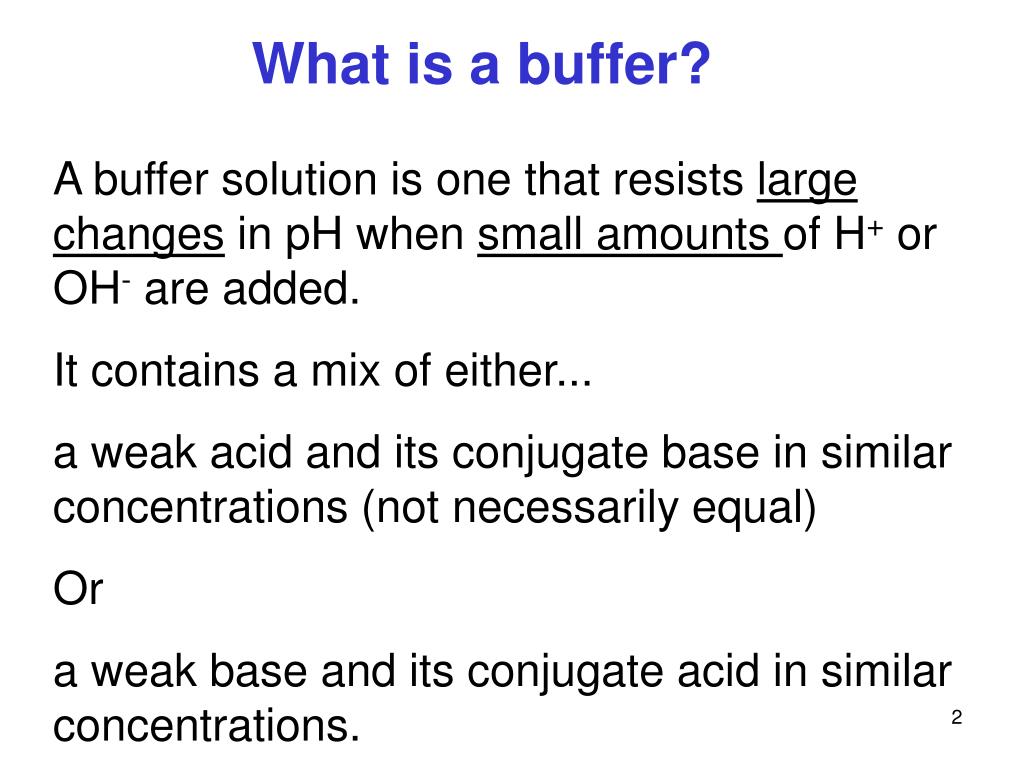
What Is A Buffer Acid . a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. what is an acidic buffer? a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A ph buffer is a solution which resists ph change when acid or base. Firstly, we need to define what is a ph buffer.
from www.slideserve.com
A ph buffer is a solution which resists ph change when acid or base. what is an acidic buffer? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. Firstly, we need to define what is a ph buffer. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial.
PPT Acids Bases Equilibria Part V Buffers PowerPoint Presentation
What Is A Buffer Acid a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. what is an acidic buffer? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A ph buffer is a solution which resists ph change when acid or base. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Firstly, we need to define what is a ph buffer.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium What Is A Buffer Acid A ph buffer is a solution which resists ph change when acid or base. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion.. What Is A Buffer Acid.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Is A Buffer Acid This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. what is an acidic buffer? A ph buffer is a solution which resists ph change when acid or base. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a.. What Is A Buffer Acid.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Acid a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. This characteristic makes buffers important in biological and chemical applications where ph. What Is A Buffer Acid.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks What Is A Buffer Acid a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. what is an acidic buffer? a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes buffers important in. What Is A Buffer Acid.
From cewwcrjd.blob.core.windows.net
Biological Buffers Ph Range at Milton Clark blog What Is A Buffer Acid buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. what is an acidic buffer? a buffer is a solution that can. What Is A Buffer Acid.
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses What Is A Buffer Acid A ph buffer is a solution which resists ph change when acid or base. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate. What Is A Buffer Acid.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint What Is A Buffer Acid a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. This characteristic makes buffers important in biological and chemical applications. What Is A Buffer Acid.
From www.youtube.com
What is buffer types of buffer Acidic buffer and basic buffer What Is A Buffer Acid A ph buffer is a solution which resists ph change when acid or base. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Firstly, we need to define what is a ph buffer. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that. What Is A Buffer Acid.
From www.youtube.com
Buffer capacity Acids and bases AP Chemistry Khan Academy YouTube What Is A Buffer Acid A ph buffer is a solution which resists ph change when acid or base. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. a mixture of a weak acid and. What Is A Buffer Acid.
From courses.lumenlearning.com
Buffers Chemistry for Majors What Is A Buffer Acid a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. what is an acidic buffer? a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. This characteristic makes buffers important in. What Is A Buffer Acid.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 What Is A Buffer Acid what is an acidic buffer? buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. A ph buffer is a solution. What Is A Buffer Acid.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation, free What Is A Buffer Acid buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. A ph buffer is a solution which resists ph change when acid or base. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Firstly, we need. What Is A Buffer Acid.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Is A Buffer Acid This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. what is an acidic buffer? a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffer, in chemistry, solution usually containing an acid and a base, or a. What Is A Buffer Acid.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance What Is A Buffer Acid what is an acidic buffer? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Firstly, we need to define what is a ph buffer. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A ph buffer is a solution which resists ph. What Is A Buffer Acid.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Is A Buffer Acid a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A ph buffer is a solution which resists ph change when acid. What Is A Buffer Acid.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download What Is A Buffer Acid A ph buffer is a solution which resists ph change when acid or base. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. Firstly, we need to define what is a ph buffer. a buffer is a solution that can resist ph change. What Is A Buffer Acid.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Acid This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid). What Is A Buffer Acid.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID4587814 What Is A Buffer Acid a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Firstly, we need to define what is a ph buffer. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that maintains the stability of a system’s ph level. What Is A Buffer Acid.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Acid Firstly, we need to define what is a ph buffer. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a.. What Is A Buffer Acid.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Is A Buffer Acid buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. a buffer is a solution that can resist ph change upon. What Is A Buffer Acid.
From www.slideserve.com
PPT Acids Bases Equilibria Part V Buffers PowerPoint Presentation What Is A Buffer Acid A ph buffer is a solution which resists ph change when acid or base. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. . What Is A Buffer Acid.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your What Is A Buffer Acid This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A ph buffer is a solution which resists ph change when acid or base. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. what is an acidic buffer? a. What Is A Buffer Acid.
From www.slideserve.com
PPT Weak acids and bases Salt of weak acid and bases buffer Lecture 9 What Is A Buffer Acid buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. what is an acidic buffer? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A ph buffer is a solution which resists ph change when. What Is A Buffer Acid.
From www.slideshare.net
Buffer system What Is A Buffer Acid A ph buffer is a solution which resists ph change when acid or base. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids. What Is A Buffer Acid.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download What Is A Buffer Acid A ph buffer is a solution which resists ph change when acid or base. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution. What Is A Buffer Acid.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide What Is A Buffer Acid buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid). What Is A Buffer Acid.
From www.sliderbase.com
Buffers Presentation Chemistry What Is A Buffer Acid what is an acidic buffer? buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. a mixture of a weak. What Is A Buffer Acid.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Is A Buffer Acid what is an acidic buffer? a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. This characteristic makes buffers. What Is A Buffer Acid.
From www.seachem.com
Seachem Acid Buffer What Is A Buffer Acid what is an acidic buffer? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities. What Is A Buffer Acid.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint What Is A Buffer Acid buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. what is an acidic buffer? This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. a buffer is a solution that can resist ph change upon the addition of an. What Is A Buffer Acid.
From www.slideserve.com
PPT Chapter 16 Aqueous Ionic Equilibrium PowerPoint Presentation What Is A Buffer Acid Firstly, we need to define what is a ph buffer. what is an acidic buffer? buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A ph buffer is a solution. What Is A Buffer Acid.
From acidsandbasesfordummieschem.weebly.com
Buffers Acid and Bases for Dummies! What Is A Buffer Acid This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A ph buffer is a solution which resists ph change when acid or base. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. what is an acidic buffer?. What Is A Buffer Acid.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download What Is A Buffer Acid This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A ph buffer is a solution which resists ph change when acid or base. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffer, in chemistry, solution usually containing an acid and a. What Is A Buffer Acid.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog What Is A Buffer Acid a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. a mixture of a weak acid and its conjugate base (or. What Is A Buffer Acid.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 What Is A Buffer Acid a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. This characteristic makes buffers important in biological and chemical applications. What Is A Buffer Acid.