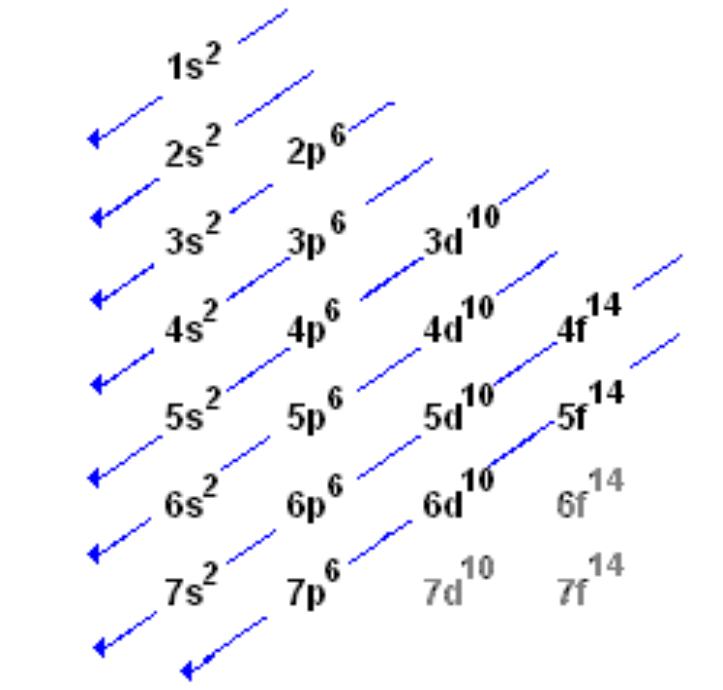
Orbital Shells Chemistry . — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. These shells are defined by the principal quantum number, given. as per the bohr model, electrons are arranged in shells. — the total nodes of an orbital is the sum of angular and radial nodes and is given in terms of the \(n\) and \(l\) quantum number by the. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams.
from antranik.org
— the total nodes of an orbital is the sum of angular and radial nodes and is given in terms of the \(n\) and \(l\) quantum number by the. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. as per the bohr model, electrons are arranged in shells. These shells are defined by the principal quantum number, given. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied.
Electrons shells and orbitals
Orbital Shells Chemistry — the total nodes of an orbital is the sum of angular and radial nodes and is given in terms of the \(n\) and \(l\) quantum number by the. — the total nodes of an orbital is the sum of angular and radial nodes and is given in terms of the \(n\) and \(l\) quantum number by the. These shells are defined by the principal quantum number, given. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. as per the bohr model, electrons are arranged in shells. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons.
From www.youtube.com
Relationship of Quantum shells, Subshells, Orbitals & Electrons Orbital Shells Chemistry — the total nodes of an orbital is the sum of angular and radial nodes and is given in terms of the \(n\) and \(l\) quantum number by the. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. — different shells contain different numbers and kinds. Orbital Shells Chemistry.
From schematicfixashiver.z21.web.core.windows.net
Orbital Diagrams Chemistry Orbital Shells Chemistry as per the bohr model, electrons are arranged in shells. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. different shells contain different numbers and kinds. Orbital Shells Chemistry.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts Orbital Shells Chemistry different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. as per the bohr model, electrons are arranged in shells. — the total nodes of an. Orbital Shells Chemistry.
From mavink.com
Electron Orbitals Explained Orbital Shells Chemistry as per the bohr model, electrons are arranged in shells. — the total nodes of an orbital is the sum of angular and radial nodes and is given in terms of the \(n\) and \(l\) quantum number by the. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be. Orbital Shells Chemistry.
From byjus.com
Orbitals Chemistry (Shapes of Atomic Orbitals) Shape of s, p, d, and Orbital Shells Chemistry revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. as per the bohr model, electrons are arranged in shells. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. different shells contain different. Orbital Shells Chemistry.
From socratic.org
What are s,p,d,f orbitals? Socratic Orbital Shells Chemistry These shells are defined by the principal quantum number, given. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. as per the bohr model, electrons are arranged in shells. — different shells contain different numbers and kinds of orbitals, and each orbital within. Orbital Shells Chemistry.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica Orbital Shells Chemistry — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by. Orbital Shells Chemistry.
From www.britannica.com
Orbital Chemistry, Physics & Applications Britannica Orbital Shells Chemistry — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. as per the bohr model, electrons are arranged in shells. These shells are defined by the principal quantum number, given. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be. Orbital Shells Chemistry.
From courses.lumenlearning.com
Organization of Electrons in Atoms Introductory Chemistry Orbital Shells Chemistry revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. — the total nodes of an orbital is the sum of angular and radial nodes and is given in terms of the \(n\) and \(l\) quantum number by the. different shells contain different numbers. Orbital Shells Chemistry.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills Orbital Shells Chemistry — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. — different shells contain different numbers and kinds of orbitals, and each orbital within. Orbital Shells Chemistry.
From www.youtube.com
Shells, Sub shells and Orbitals AS level Chemistry Notes YouTube Orbital Shells Chemistry revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. These shells are defined by the principal quantum number, given. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. — each shell is subdivided. Orbital Shells Chemistry.
From chem.libretexts.org
3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts Orbital Shells Chemistry — the total nodes of an orbital is the sum of angular and radial nodes and is given in terms of the \(n\) and \(l\) quantum number by the. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. These shells are defined by the principal quantum number,. Orbital Shells Chemistry.
From www.youtube.com
What are shells,subshells and orbitals? Difference between shells Orbital Shells Chemistry as per the bohr model, electrons are arranged in shells. — the total nodes of an orbital is the sum of angular and radial nodes and is given in terms of the \(n\) and \(l\) quantum number by the. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be. Orbital Shells Chemistry.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Orbital Shells Chemistry as per the bohr model, electrons are arranged in shells. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. — the. Orbital Shells Chemistry.
From www.stonecoldhands.com
Electron Shells and Orbitals Stone Cold Chemistry Talk Orbital Shells Chemistry different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. as per the bohr model, electrons are arranged in shells. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. These shells are defined by the principal. Orbital Shells Chemistry.
From guweb2.gonzaga.edu
CHEM 101 Lecture 7 Orbital Shells Chemistry These shells are defined by the principal quantum number, given. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. as per the bohr model, electrons are arranged in shells. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons. Orbital Shells Chemistry.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent Orbital Shells Chemistry different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. as per the bohr model, electrons are arranged in shells. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. — the total nodes of an. Orbital Shells Chemistry.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent Orbital Shells Chemistry — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. Orbital Shells Chemistry.
From www.youtube.com
Shells, Subshells, and Orbitals l Understand the difference YouTube Orbital Shells Chemistry as per the bohr model, electrons are arranged in shells. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. These shells are defined by the principal quantum number, given. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be. Orbital Shells Chemistry.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Orbital Shells Chemistry — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. as per the bohr model, electrons are arranged in shells. different shells contain. Orbital Shells Chemistry.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Orbital Shells Chemistry — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. These shells are defined by the principal quantum number, given. — the total nodes of an orbital. Orbital Shells Chemistry.
From diagramdataconfusion.z22.web.core.windows.net
Electron Configuration And Orbital Diagram Orbital Shells Chemistry These shells are defined by the principal quantum number, given. — the total nodes of an orbital is the sum of angular and radial nodes and is given in terms of the \(n\) and \(l\) quantum number by the. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with. Orbital Shells Chemistry.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills Orbital Shells Chemistry — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. These shells are defined by the principal quantum number, given. as per the bohr model, electrons are arranged in shells. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the. Orbital Shells Chemistry.
From chemistrytalk.org
Electron Orbitals & Orbital Shapes ChemTalk Orbital Shells Chemistry revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. These shells are defined by the principal quantum number, given. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. — each shell is subdivided. Orbital Shells Chemistry.
From lavelle.chem.ucla.edu
subshells and orbitals CHEMISTRY COMMUNITY Orbital Shells Chemistry revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. — the total nodes of an orbital is the sum of angular and radial nodes and is given in terms of the \(n\) and \(l\) quantum number by the. — each shell is subdivided. Orbital Shells Chemistry.
From ar.inspiredpencil.com
Electron Orbital Shells Orbital Shells Chemistry different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell. Orbital Shells Chemistry.
From socratic.org
What is the difference between electron shells and electron orbitals Orbital Shells Chemistry revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. different shells contain different numbers and kinds of orbitals, and each orbital within a. Orbital Shells Chemistry.
From antranik.org
Electrons shells and orbitals Orbital Shells Chemistry — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. These shells are defined by the principal quantum number, given. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. different shells contain different numbers and kinds of. Orbital Shells Chemistry.
From www.britannica.com
Chemical bonding Atomic Orbitals, Shapes, Hybridization Britannica Orbital Shells Chemistry These shells are defined by the principal quantum number, given. as per the bohr model, electrons are arranged in shells. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. — the total nodes of an orbital is the sum of angular and radial nodes and is. Orbital Shells Chemistry.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Orbital Shells Chemistry — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. These shells are defined by the principal quantum number, given. — the total nodes of an orbital. Orbital Shells Chemistry.
From www.vedantu.com
Define an atomic orbital. Orbital Shells Chemistry — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. These shells are defined by the principal quantum number, given. — the total nodes of an orbital is the sum of angular and radial nodes and is given in terms of the \(n\) and \(l\) quantum number by. Orbital Shells Chemistry.
From chem.libretexts.org
6.6 3D Representation of Orbitals Chemistry LibreTexts Orbital Shells Chemistry revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. These shells are defined by the principal quantum number, given. as per the. Orbital Shells Chemistry.
From www.youtube.com
OCR AS Chemistry Unit F321, Module 2 Shells, sub shells and orbitals Orbital Shells Chemistry — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied by two electrons. — each shell is subdivided into subshells, which are made up of orbitals, each of which has. Orbital Shells Chemistry.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Orbital Shells Chemistry — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. These shells are defined by the principal quantum number, given. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. as per the bohr model, electrons are arranged. Orbital Shells Chemistry.
From www.youtube.com
Class 11th Chemistry Concept of shell, sub shell & orbitals YouTube Orbital Shells Chemistry These shells are defined by the principal quantum number, given. — different shells contain different numbers and kinds of orbitals, and each orbital within a shell can be occupied. — each shell is subdivided into subshells, which are made up of orbitals, each of which has electrons with different. as per the bohr model, electrons are arranged. Orbital Shells Chemistry.