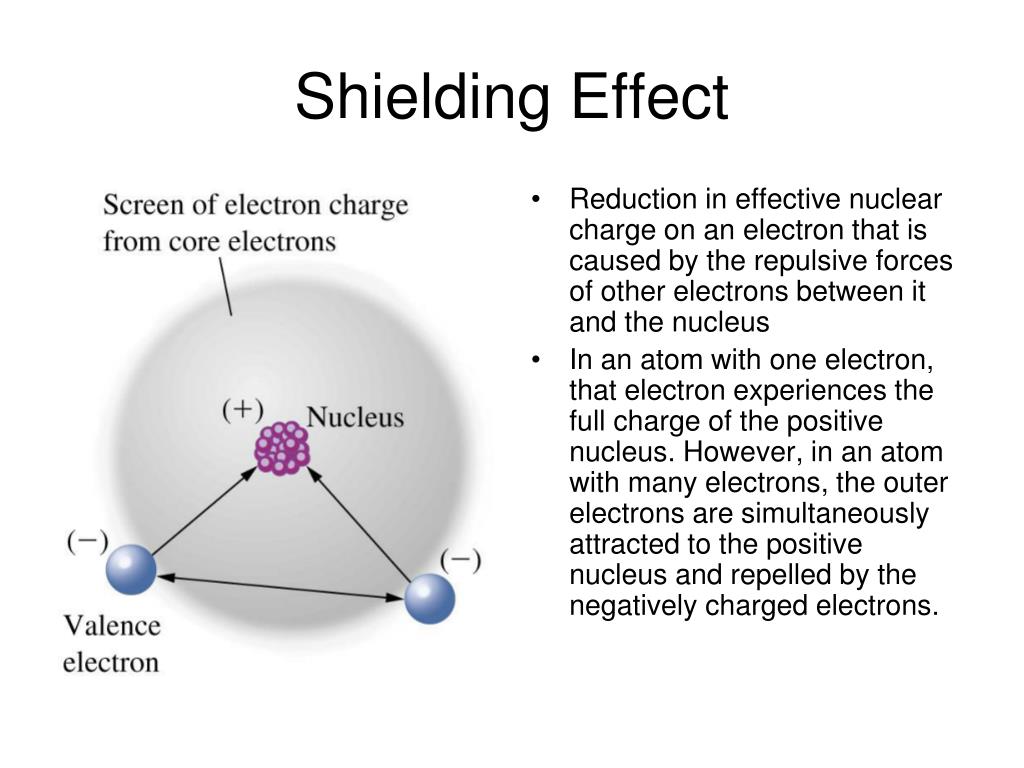
What Is Shielding Effect Of Class 11 . The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. What is the shielding effect? The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. In an atom, electrons can shield each other from the pull of the nucleus. This effect, called the shielding effect, describes the decrease in attraction between an electron. When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. The screening or shielding effect: It implies that the shielding.
from ar.inspiredpencil.com
It implies that the shielding. What is the shielding effect? The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. In an atom, electrons can shield each other from the pull of the nucleus. The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. The screening or shielding effect: This effect, called the shielding effect, describes the decrease in attraction between an electron.
Shielding Effect Electrons
What Is Shielding Effect Of Class 11 It implies that the shielding. In an atom, electrons can shield each other from the pull of the nucleus. What is the shielding effect? It implies that the shielding. The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. This effect, called the shielding effect, describes the decrease in attraction between an electron. The screening or shielding effect: The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is.
From www.youtube.com
Shielding effect Class 11 YouTube What Is Shielding Effect Of Class 11 The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. It implies that the shielding. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons. What Is Shielding Effect Of Class 11.
From www.youtube.com
SHIELDING/SCREENING EFFECT YouTube What Is Shielding Effect Of Class 11 In an atom, electrons can shield each other from the pull of the nucleus. This effect, called the shielding effect, describes the decrease in attraction between an electron. The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. It implies that the shielding. The screening or shielding effect: When. What Is Shielding Effect Of Class 11.
From www.youtube.com
screening or shielding effect //shielding and screening effect What Is Shielding Effect Of Class 11 The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. It implies that the shielding. What is the shielding effect? This effect, called the shielding effect, describes the decrease in attraction between an electron. In an atom, electrons can shield each other from the. What Is Shielding Effect Of Class 11.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick What Is Shielding Effect Of Class 11 In an atom, electrons can shield each other from the pull of the nucleus. It implies that the shielding. The screening or shielding effect: This effect, called the shielding effect, describes the decrease in attraction between an electron. What is the shielding effect? The decrease in the force of attraction exerted by the nucleus on the valence electrons due to. What Is Shielding Effect Of Class 11.
From ar.inspiredpencil.com
Beta Particles What Is Shielding Effect Of Class 11 What is the shielding effect? The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. It implies that the shielding. The screening or shielding effect: This effect, called the shielding effect, describes the decrease in attraction. What Is Shielding Effect Of Class 11.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding Effect Of Class 11 It implies that the shielding. This effect, called the shielding effect, describes the decrease in attraction between an electron. The screening or shielding effect: The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. When the number of inner electrons is greater, they shelter the outermost electron from the. What Is Shielding Effect Of Class 11.
From mungfali.com
What Is Shielding Effect What Is Shielding Effect Of Class 11 When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. The screening or shielding effect: It implies that the shielding. This effect, called the shielding effect, describes the decrease in attraction between an electron. In an atom, electrons can shield each other from the pull of the nucleus. The decrease in the force of. What Is Shielding Effect Of Class 11.
From www.youtube.com
What is Shielding Effect in Simple Words Shielding Effect Definition What Is Shielding Effect Of Class 11 What is the shielding effect? The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. The screening or shielding effect: It implies that the shielding. In an atom, electrons can shield each other from the pull of the nucleus. This effect, called the shielding effect, describes the decrease in attraction between an. What Is Shielding Effect Of Class 11.
From www.quanswer.com
What is shielding and deshielding effect? (1&? Quanswer What Is Shielding Effect Of Class 11 The screening or shielding effect: When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. It implies that the shielding. What is the shielding effect? The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. In an atom, electrons can shield each other from the pull. What Is Shielding Effect Of Class 11.
From siliconvlsi.com
Types of Shielding in VLSI Siliconvlsi What Is Shielding Effect Of Class 11 What is the shielding effect? The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. It implies that the shielding. This effect, called the shielding effect, describes the. What Is Shielding Effect Of Class 11.
From www.youtube.com
Chemistry Class 9 Ch 3 Shielding effect Spectrum of Knowledge YouTube What Is Shielding Effect Of Class 11 It implies that the shielding. The screening or shielding effect: In an atom, electrons can shield each other from the pull of the nucleus. When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. What is the shielding effect? The decrease in the force of attraction exerted by the nucleus on the valence electrons. What Is Shielding Effect Of Class 11.
From scienceinfo.com
Shielding effect What Is Shielding Effect Of Class 11 The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is.. What Is Shielding Effect Of Class 11.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is Shielding Effect Of Class 11 This effect, called the shielding effect, describes the decrease in attraction between an electron. It implies that the shielding. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. The attraction between outer electrons and the nucleus decreases as the number of electrons between. What Is Shielding Effect Of Class 11.
From www.youtube.com
Shielding and Deshielding Effect/ Shielding and Deshielding effect in What Is Shielding Effect Of Class 11 The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. The screening or shielding effect: When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the. What Is Shielding Effect Of Class 11.
From chemisfast.blogspot.com
Lanthanide contractiondefinitioncausesconsequences in chemistry PG What Is Shielding Effect Of Class 11 The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. What is the shielding effect? It implies that the shielding. This effect, called the shielding effect, describes the decrease in attraction. What Is Shielding Effect Of Class 11.
From www.youtube.com
What is a shielding effect ? Its Trends in periodic table Chemistry What Is Shielding Effect Of Class 11 The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. It implies that the shielding. The decrease in the force of attraction exerted by the nucleus on the valence electrons due. What Is Shielding Effect Of Class 11.
From ecurrencythailand.com
What Is The Electron Shielding Effect? Best 7 Answer What Is Shielding Effect Of Class 11 What is the shielding effect? The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. The screening or shielding effect: When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. The decrease in the force of attraction exerted by the nucleus on the. What Is Shielding Effect Of Class 11.
From snappernews.com
What Is Screening Effect? SnapperNews What Is Shielding Effect Of Class 11 In an atom, electrons can shield each other from the pull of the nucleus. It implies that the shielding. This effect, called the shielding effect, describes the decrease in attraction between an electron. What is the shielding effect? The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. When. What Is Shielding Effect Of Class 11.
From giombptgj.blob.core.windows.net
Which Element Has The Highest Shielding Effect at Victoria Hoover blog What Is Shielding Effect Of Class 11 In an atom, electrons can shield each other from the pull of the nucleus. The screening or shielding effect: When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. The decrease in the. What Is Shielding Effect Of Class 11.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free What Is Shielding Effect Of Class 11 In an atom, electrons can shield each other from the pull of the nucleus. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. What is the shielding effect? The attraction between outer electrons and the nucleus decreases as the number of electrons between. What Is Shielding Effect Of Class 11.
From www.youtube.com
shielding effect chemistry class 11 in hindiस्क्रीनिंग इफ़ेक्ट हिंदी What Is Shielding Effect Of Class 11 In an atom, electrons can shield each other from the pull of the nucleus. It implies that the shielding. What is the shielding effect? When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. The screening or shielding effect: The shielding effect is defined as the ability of electrons in an atom to protect. What Is Shielding Effect Of Class 11.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID6535293 What Is Shielding Effect Of Class 11 The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. It implies that the shielding. The screening or shielding effect: What is the shielding effect? The shielding effect is defined as the ability of electrons in an atom to protect one another from the. What Is Shielding Effect Of Class 11.
From animalia-life.club
Shielding Effect Trend What Is Shielding Effect Of Class 11 The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. The screening or shielding effect: It implies that the shielding. What is the shielding effect? In an atom, electrons can shield. What Is Shielding Effect Of Class 11.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube What Is Shielding Effect Of Class 11 The screening or shielding effect: What is the shielding effect? The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. It implies that the shielding. In an atom, electrons can shield each other from the pull of the nucleus. The decrease in the force of attraction exerted by the. What Is Shielding Effect Of Class 11.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting What Is Shielding Effect Of Class 11 The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is.. What Is Shielding Effect Of Class 11.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting What Is Shielding Effect Of Class 11 When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. The screening or shielding effect: What is the shielding effect? It implies that the shielding. This effect, called the shielding effect, describes the decrease in attraction between an electron. The decrease in the force of attraction exerted by the nucleus on the valence electrons. What Is Shielding Effect Of Class 11.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The What Is Shielding Effect Of Class 11 The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. The screening or shielding effect: It implies that the shielding. In an atom, electrons can shield each other from the pull of the nucleus. What is the shielding effect? When the number of inner electrons is greater, they shelter. What Is Shielding Effect Of Class 11.
From byjus.com
WHAT IS SHIELDING EFFECT?WHAT IS THE VALUE OF SHIELDING CONSTANT AND What Is Shielding Effect Of Class 11 This effect, called the shielding effect, describes the decrease in attraction between an electron. What is the shielding effect? The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. The shielding effect is defined as the ability of electrons in an atom to protect one another from the nucleus's pull. The decrease. What Is Shielding Effect Of Class 11.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is Shielding Effect Of Class 11 The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. It implies that the shielding. The shielding effect is defined as the ability of electrons in. What Is Shielding Effect Of Class 11.
From byjus.com
What is effective nuclear charge and shielding effect? What Is Shielding Effect Of Class 11 It implies that the shielding. In an atom, electrons can shield each other from the pull of the nucleus. This effect, called the shielding effect, describes the decrease in attraction between an electron. When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. The decrease in the force of attraction exerted by the nucleus. What Is Shielding Effect Of Class 11.
From www.differencebetween.com
What is the Difference Between Effective Nuclear Charge and Shielding What Is Shielding Effect Of Class 11 What is the shielding effect? The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. It implies that the shielding. The shielding effect is defined as the ability of electrons in an atom to protect one. What Is Shielding Effect Of Class 11.
From www.youtube.com
🔴 Energies of Orbitals Shielding Effect and Degenerate Orbitals What Is Shielding Effect Of Class 11 The screening or shielding effect: The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. In an atom, electrons can shield each other from the pull of the nucleus. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the. What Is Shielding Effect Of Class 11.
From www.youtube.com
What is Shielding Effect? shielding Effect বলতে কি বোঝ? Class 11 What Is Shielding Effect Of Class 11 What is the shielding effect? The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. The screening or shielding effect: The decrease in the force of attraction exerted by the nucleus on the valence electrons due. What Is Shielding Effect Of Class 11.
From www.youtube.com
Periodic Classification Of Elements L5 Screening Effect Shielding What Is Shielding Effect Of Class 11 This effect, called the shielding effect, describes the decrease in attraction between an electron. The screening or shielding effect: In an atom, electrons can shield each other from the pull of the nucleus. The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. It implies that the shielding. What is the shielding. What Is Shielding Effect Of Class 11.
From www.teachoo.com
[Term 2] Account for (b) In case of transition elements, ions of same What Is Shielding Effect Of Class 11 The attraction between outer electrons and the nucleus decreases as the number of electrons between them and the. In an atom, electrons can shield each other from the pull of the nucleus. When the number of inner electrons is greater, they shelter the outermost electron from the nucleus,. What is the shielding effect? The decrease in the force of attraction. What Is Shielding Effect Of Class 11.