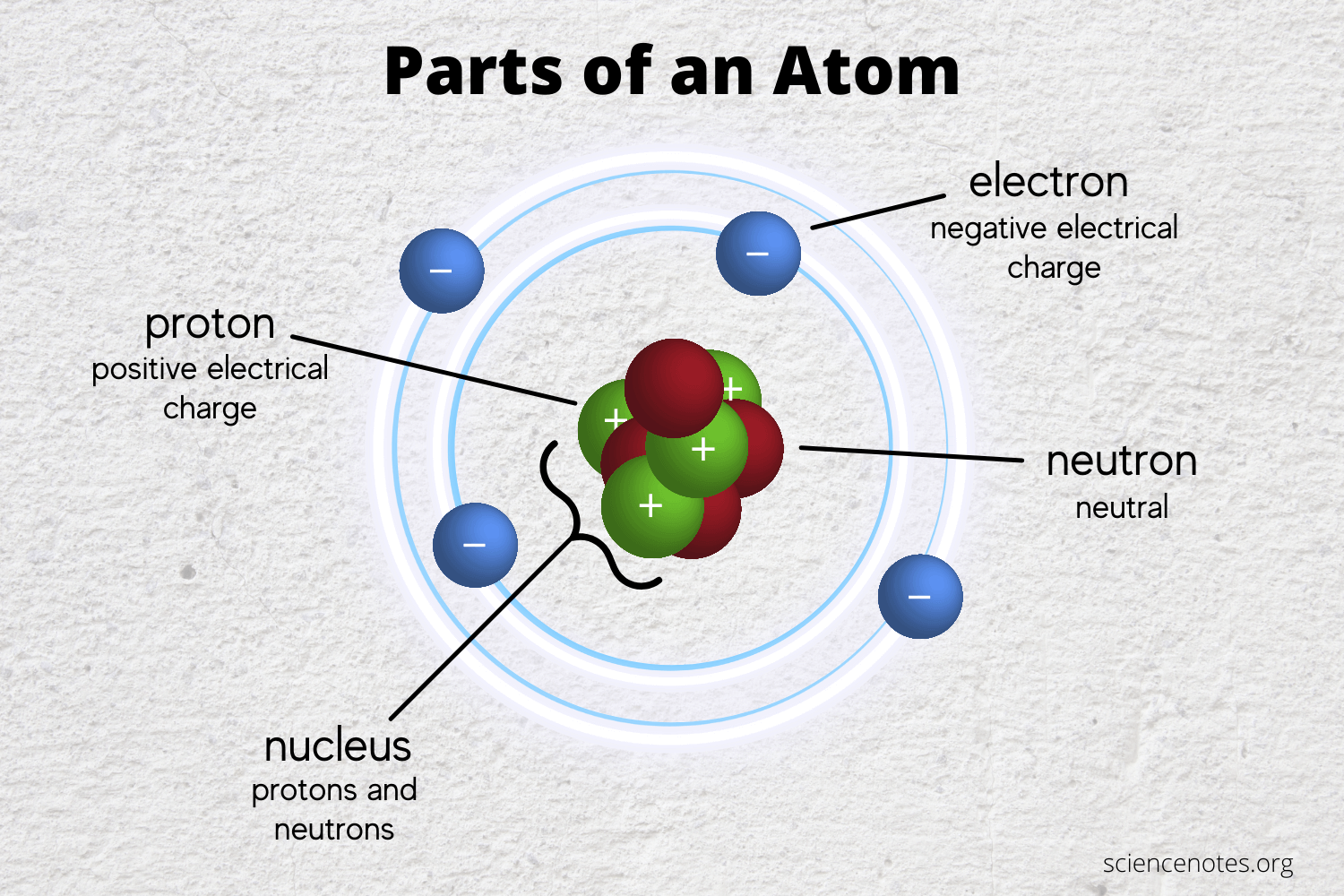
Do Atoms Have More Electrons Than Protons . If there is an equal. watch this video to learn how protons, neutrons, and electrons are arranged in. protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. atoms do not always contain the same number of electrons and protons, although this state is common. You can find the number of neutrons if. if the charge is positive, there are more protons than electrons. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. If the charge is negative, electrons are in excess. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. Protons have a positive charge, and electrons have a negative charge.
from www.mindomo.com
all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. You can find the number of neutrons if. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. watch this video to learn how protons, neutrons, and electrons are arranged in. Protons have a positive charge, and electrons have a negative charge. If the charge is negative, electrons are in excess. protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. atoms do not always contain the same number of electrons and protons, although this state is common. if the charge is positive, there are more protons than electrons. If there is an equal.
Unit 1 Matter And Periodic Table Trends Mind Map
Do Atoms Have More Electrons Than Protons If the charge is negative, electrons are in excess. If the charge is negative, electrons are in excess. You can find the number of neutrons if. Protons have a positive charge, and electrons have a negative charge. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. If there is an equal. if the charge is positive, there are more protons than electrons. protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. atoms do not always contain the same number of electrons and protons, although this state is common. watch this video to learn how protons, neutrons, and electrons are arranged in.
From mydiagram.online
[DIAGRAM] Gold Electron Diagram With Science Do Atoms Have More Electrons Than Protons if the charge is positive, there are more protons than electrons. watch this video to learn how protons, neutrons, and electrons are arranged in. If there is an equal. protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. all atoms have the same. Do Atoms Have More Electrons Than Protons.
From www.nagwa.com
Question Video Comparing a Positive Ion to a Neutral Atom Nagwa Do Atoms Have More Electrons Than Protons If the charge is negative, electrons are in excess. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. Protons have a positive charge, and electrons have. Do Atoms Have More Electrons Than Protons.
From spmchemistry.blog.onlinetuition.com.my
Modern Atomic Model SPM Chemistry Do Atoms Have More Electrons Than Protons You can find the number of neutrons if. If the charge is negative, electrons are in excess. protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. atoms do not always contain the same number of electrons and protons, although this state is common. If there. Do Atoms Have More Electrons Than Protons.
From ircamera.as.arizona.edu
Spectroscopy Do Atoms Have More Electrons Than Protons watch this video to learn how protons, neutrons, and electrons are arranged in. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. atoms do not always contain the same number of electrons and protons, although this state is common. all atoms have the same number of. Do Atoms Have More Electrons Than Protons.
From www.youtube.com
What are Protons, Neutrons, and Electrons? How are they Arranged Inside Do Atoms Have More Electrons Than Protons If there is an equal. You can find the number of neutrons if. watch this video to learn how protons, neutrons, and electrons are arranged in. protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. Protons have a positive charge, and electrons have a negative. Do Atoms Have More Electrons Than Protons.
From www.slideserve.com
PPT Atoms, Molecules & Ions PowerPoint Presentation, free download Do Atoms Have More Electrons Than Protons If there is an equal. You can find the number of neutrons if. atoms do not always contain the same number of electrons and protons, although this state is common. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. protons and neutrons have approximately the same mass,. Do Atoms Have More Electrons Than Protons.
From www.broadlearnings.com
Atomic Structure Broad Learnings Do Atoms Have More Electrons Than Protons atoms do not always contain the same number of electrons and protons, although this state is common. Protons have a positive charge, and electrons have a negative charge. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. if the charge is positive, there are more protons than. Do Atoms Have More Electrons Than Protons.
From www.pinterest.com
Oxygen shells Oxygen, Atom, Chemistry projects Do Atoms Have More Electrons Than Protons all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. You can find the number of neutrons if. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. protons and neutrons have approximately the same mass, but they are. Do Atoms Have More Electrons Than Protons.
From room201csa.weebly.com
Atoms and Elements Científicos Matemáticos Do Atoms Have More Electrons Than Protons all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. If there is an equal. atoms do not always contain the same number of electrons and protons, although this state is common. if the charge is positive, there are more protons than electrons. If the charge is negative,. Do Atoms Have More Electrons Than Protons.
From users.highland.edu
Lewis Structures and Covalent Bonding Do Atoms Have More Electrons Than Protons If there is an equal. if the charge is positive, there are more protons than electrons. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. Protons have a positive charge, and electrons have a negative charge. If the charge is negative, electrons are in excess. protons and. Do Atoms Have More Electrons Than Protons.
From chemistry.about.com
Basic Model of the Atom Atomic Theory Do Atoms Have More Electrons Than Protons If there is an equal. atoms do not always contain the same number of electrons and protons, although this state is common. If the charge is negative, electrons are in excess. watch this video to learn how protons, neutrons, and electrons are arranged in. Protons have a positive charge, and electrons have a negative charge. You can find. Do Atoms Have More Electrons Than Protons.
From www.askiitians.com
Introduction of Atoms Free Online Study Material & Videos askIITians Do Atoms Have More Electrons Than Protons atoms do not always contain the same number of electrons and protons, although this state is common. Protons have a positive charge, and electrons have a negative charge. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. if the charge is positive, there are more protons than. Do Atoms Have More Electrons Than Protons.
From www.pinterest.com
Protons Neutrons and Electrons Protons, Electrons, Neutrons Do Atoms Have More Electrons Than Protons Protons have a positive charge, and electrons have a negative charge. You can find the number of neutrons if. if the charge is positive, there are more protons than electrons. If the charge is negative, electrons are in excess. atoms do not always contain the same number of electrons and protons, although this state is common. If there. Do Atoms Have More Electrons Than Protons.
From www.electronicsandyou.com
Structure of an Atom Structure & Use of Electron & Proton in Electronics Do Atoms Have More Electrons Than Protons If the charge is negative, electrons are in excess. protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. atoms do not always contain the same number of electrons and protons, although this state is common. Protons have a positive charge, and electrons have a negative. Do Atoms Have More Electrons Than Protons.
From www.elephango.com
Negative Electrons Educational Resources K12 Learning, Chemistry Do Atoms Have More Electrons Than Protons atoms do not always contain the same number of electrons and protons, although this state is common. Protons have a positive charge, and electrons have a negative charge. if the charge is positive, there are more protons than electrons. protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately. Do Atoms Have More Electrons Than Protons.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic Do Atoms Have More Electrons Than Protons watch this video to learn how protons, neutrons, and electrons are arranged in. protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. If the charge is negative, electrons are in excess. If there is an equal. all atoms have the same number of electrons. Do Atoms Have More Electrons Than Protons.
From www.scienceabc.com
Isotopes Definition, Explanation, Properties And Examples Do Atoms Have More Electrons Than Protons protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. If there is an equal. atoms do not always contain the same number of electrons and. Do Atoms Have More Electrons Than Protons.
From www.nagwa.com
Question Video Determining If Two Atoms Are Identical Given the Number Do Atoms Have More Electrons Than Protons all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. If the charge is negative, electrons are in excess. atoms do not always contain the same number of electrons and protons, although this state is common. if the charge is positive, there are more protons than electrons. . Do Atoms Have More Electrons Than Protons.
From wghsjuniorscience.weebly.com
Atomic structure WGHS Junior Science Do Atoms Have More Electrons Than Protons all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000. Do Atoms Have More Electrons Than Protons.
From donavan-has-beck.blogspot.com
Describe How Changing the Particles Changed the Atom DonavanhasBeck Do Atoms Have More Electrons Than Protons protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. watch this video to learn how protons, neutrons, and electrons are arranged in. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. You can find. Do Atoms Have More Electrons Than Protons.
From mungfali.com
Protons Neutrons And Electrons Diagram Do Atoms Have More Electrons Than Protons protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. Protons have a positive charge, and electrons have a negative charge. if the charge is positive, there are more protons than electrons. atoms do not always contain the same number of electrons and protons, although. Do Atoms Have More Electrons Than Protons.
From depositphotos.com
What Happens Atoms Bond Infographic Diagram Showing How Electrons Do Atoms Have More Electrons Than Protons Protons have a positive charge, and electrons have a negative charge. If the charge is negative, electrons are in excess. watch this video to learn how protons, neutrons, and electrons are arranged in. atoms do not always contain the same number of electrons and protons, although this state is common. You can find the number of neutrons if.. Do Atoms Have More Electrons Than Protons.
From www.youtube.com
2.1.5 Protons/neutrons/electrons in atoms/ions from mass/atomic number Do Atoms Have More Electrons Than Protons if the charge is positive, there are more protons than electrons. atoms do not always contain the same number of electrons and protons, although this state is common. If the charge is negative, electrons are in excess. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. . Do Atoms Have More Electrons Than Protons.
From www.slideserve.com
PPT How many protons, neutrons & electrons are present in atom Do Atoms Have More Electrons Than Protons watch this video to learn how protons, neutrons, and electrons are arranged in. Protons have a positive charge, and electrons have a negative charge. If there is an equal. If the charge is negative, electrons are in excess. protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times. Do Atoms Have More Electrons Than Protons.
From mungfali.com
Protons Neutrons And Electrons Diagram Do Atoms Have More Electrons Than Protons all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. Protons have a positive charge, and electrons have a negative charge. You can find the number of neutrons if. atoms do not always contain the same number of electrons and protons, although this state is common. if the. Do Atoms Have More Electrons Than Protons.
From www.thesciencehive.co.uk
Atomic Structure* — the science sauce Do Atoms Have More Electrons Than Protons atoms do not always contain the same number of electrons and protons, although this state is common. If the charge is negative, electrons are in excess. watch this video to learn how protons, neutrons, and electrons are arranged in. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. Do Atoms Have More Electrons Than Protons.
From wireenginehaymakings.z14.web.core.windows.net
Diagram Of Protons Neutrons And Electrons Do Atoms Have More Electrons Than Protons Protons have a positive charge, and electrons have a negative charge. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. You can find the number of neutrons if. If there is an equal. all atoms have the same number of electrons as protons, so the positive and negative. Do Atoms Have More Electrons Than Protons.
From slides.com
Chemistry Do Atoms Have More Electrons Than Protons If there is an equal. Protons have a positive charge, and electrons have a negative charge. watch this video to learn how protons, neutrons, and electrons are arranged in. if the charge is positive, there are more protons than electrons. You can find the number of neutrons if. all atoms have the same number of electrons as. Do Atoms Have More Electrons Than Protons.
From www.teachoo.com
Nucleons, Atomic Number and Mass Number Definition [with Examples] Do Atoms Have More Electrons Than Protons You can find the number of neutrons if. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. If there is an equal. If the charge is negative, electrons are in excess. watch this video to learn how protons, neutrons, and electrons are arranged in. protons and neutrons. Do Atoms Have More Electrons Than Protons.
From gadgetmedia.org
Discovering Subatomic Particles Proton, Neutron and Electrons Gadget Do Atoms Have More Electrons Than Protons if the charge is positive, there are more protons than electrons. Protons have a positive charge, and electrons have a negative charge. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. If there is an equal. You can find the number of neutrons if. all atoms have. Do Atoms Have More Electrons Than Protons.
From www.mindomo.com
Unit 1 Matter And Periodic Table Trends Mind Map Do Atoms Have More Electrons Than Protons protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. atoms do not always contain the same number of electrons and protons, although this state is common. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. Do Atoms Have More Electrons Than Protons.
From liveandlovescience.weebly.com
Atoms Protons, Electrons, and Neutrons Live and Love Science Do Atoms Have More Electrons Than Protons protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. You can find the number of neutrons if. If the charge is negative, electrons are in excess. If there is an equal. atoms do not always contain the same number of electrons and protons, although this. Do Atoms Have More Electrons Than Protons.
From studylib.net
atom Do Atoms Have More Electrons Than Protons If there is an equal. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. If the charge is negative, electrons are in excess. if the charge is positive,. Do Atoms Have More Electrons Than Protons.
From slidetodoc.com
Introduction to Basic Chemistry Protons Neutrons Electrons and Do Atoms Have More Electrons Than Protons You can find the number of neutrons if. protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive. If there is an equal. Protons have a positive charge, and electrons have a negative charge. watch this video to learn how protons, neutrons, and electrons are arranged. Do Atoms Have More Electrons Than Protons.
From sciencenotes.org
Are Two Atoms of the Same Element Identical? Do Atoms Have More Electrons Than Protons all atoms have the same number of electrons as protons, so the positive and negative charges cancel out, making. If the charge is negative, electrons are in excess. watch this video to learn how protons, neutrons, and electrons are arranged in. if the charge is positive, there are more protons than electrons. protons and neutrons have. Do Atoms Have More Electrons Than Protons.