Copper And Zinc Balanced Equation . For each element, we check if the number of atoms is balanced on both sides. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. How do you balance chemical equations step by step? Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Balance a chemical equation when given the unbalanced equation. Explain the roles of subscripts and coefficients in chemical equations. First, we set all coefficients to 1: 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. Balance a chemical equation when given the unbalanced equation. The balanced equation will appear. Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to.
from www.slideshare.net
Explain the roles of subscripts and coefficients in chemical equations. How do you balance chemical equations step by step? First, we set all coefficients to 1: Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. Balance a chemical equation when given the unbalanced equation. Balance a chemical equation when given the unbalanced equation. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. The balanced equation will appear. For each element, we check if the number of atoms is balanced on both sides.
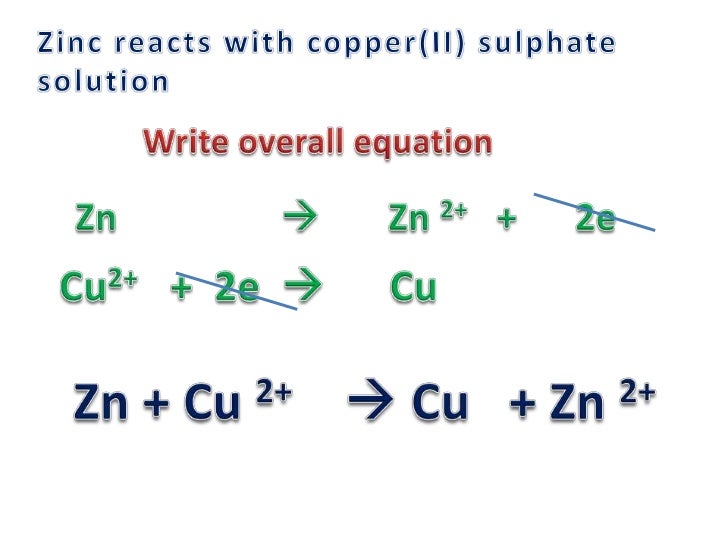
Redox= quiz part 1 with answers
Copper And Zinc Balanced Equation How do you balance chemical equations step by step? First, we set all coefficients to 1: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. For each element, we check if the number of atoms is balanced on both sides. How do you balance chemical equations step by step? Balance a chemical equation when given the unbalanced equation. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. The balanced equation will appear. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Explain the roles of subscripts and coefficients in chemical equations. Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. Explain the roles of subscripts and coefficients in chemical equations. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. Balance a chemical equation when given the unbalanced equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Copper And Zinc Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two. Copper And Zinc Balanced Equation.
From www.toppr.com
Write a balanced equation for the preparation of the following salts.(i Copper And Zinc Balanced Equation How do you balance chemical equations step by step? Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. For each element, we check if the number of atoms is balanced on both sides. First, we set all coefficients to 1: Balance a chemical equation when given the unbalanced equation. Balance a chemical equation when. Copper And Zinc Balanced Equation.
From www.toppr.com
Identify the type of reactions taking place in each of the following Copper And Zinc Balanced Equation How do you balance chemical equations step by step? Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. First, we set. Copper And Zinc Balanced Equation.
From www.slideshare.net
Replacement reactions Copper And Zinc Balanced Equation 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. Balance a chemical equation when given the unbalanced equation. For each element, we check if the number of atoms is balanced on both sides. Explain the roles of subscripts and coefficients in chemical equations. To balance a chemical equation, enter an equation of a chemical reaction and. Copper And Zinc Balanced Equation.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Copper And Zinc Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. First, we set all coefficients to 1: 1 zn + 1 cuso 4 = 1 znso 4. Copper And Zinc Balanced Equation.
From www.numerade.com
Using the activity series (Table 4.5), write balanced chemical Copper And Zinc Balanced Equation 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Explain the roles of subscripts and coefficients in chemical equations. How do you balance chemical equations step by step? The chemical equation described in section. Copper And Zinc Balanced Equation.
From www.coursehero.com
[Solved] link for the experiment https//youtu.be/ILJhI43wVA Part Copper And Zinc Balanced Equation Balance a chemical equation when given the unbalanced equation. Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. Balance a chemical equation when given the unbalanced equation. How do you balance chemical equations step by step? For each element, we check if the number of atoms is balanced on both sides. The balanced equation. Copper And Zinc Balanced Equation.
From www.numerade.com
SOLVED B) zinc and copper(II) sulfate Obs Reagents And Srav Products Copper And Zinc Balanced Equation Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. The balanced equation will appear. For each element, we check if the number of atoms is balanced on both sides. How do you balance chemical equations step by step? The chemical equation described in section 4.1. Copper And Zinc Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equations 1 zinc react with Copper And Zinc Balanced Equation How do you balance chemical equations step by step? First, we set all coefficients to 1: Balance a chemical equation when given the unbalanced equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. For each element,. Copper And Zinc Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the singlereplacement oxidation Copper And Zinc Balanced Equation For each element, we check if the number of atoms is balanced on both sides. The balanced equation will appear. How do you balance chemical equations step by step? The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. 1 zn + 1 cuso 4 = 1. Copper And Zinc Balanced Equation.
From www.youtube.com
How to Balance Zn + CuSO4 = Cu + ZnSO4 (Zinc plus Copper (II) Sulfate Copper And Zinc Balanced Equation For each element, we check if the number of atoms is balanced on both sides. Explain the roles of subscripts and coefficients in chemical equations. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. First, we set all coefficients to 1: Balance a chemical equation. Copper And Zinc Balanced Equation.
From www.toppr.com
Write balanced chemical equations the following word equationZinc Copper And Zinc Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. First, we set all coefficients to 1: Balance a chemical equation when given the unbalanced equation. How do you balance chemical equations step by step? Let us use a double displacement reaction of lead (ii) nitrate and potassium. Copper And Zinc Balanced Equation.
From www.chegg.com
Solved Write A Balanced Chemical Equation For Each Reacti... Copper And Zinc Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. How do you balance chemical equations step by step? For each element, we check if the number of atoms is balanced on both sides. The chemical. Copper And Zinc Balanced Equation.
From studentcomfort26.gitlab.io
Top Notch Percent Yield Of A Reaction Calculator Chemical Equilibrium Copper And Zinc Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Explain the roles of subscripts and coefficients in chemical equations. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. For each element, we check if the number of atoms is balanced on both sides. 1 zn + 1 cuso 4 = 1. Copper And Zinc Balanced Equation.
From keplarllp.com
😀 Zn cuso4 net ionic equation. Help writing complete ionic and net Copper And Zinc Balanced Equation Balance a chemical equation when given the unbalanced equation. The balanced equation will appear. First, we set all coefficients to 1: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. For each element, we check if the number of. Copper And Zinc Balanced Equation.
From www.chegg.com
Solved 761. Zinc metal reacts with aqueous copper(II) Copper And Zinc Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. For each element, we check. Copper And Zinc Balanced Equation.
From www.numerade.com
13. Write a balanced equation for the single displacement reaction for Copper And Zinc Balanced Equation Balance a chemical equation when given the unbalanced equation. Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. The balanced equation will appear. For each element, we check if the number of atoms is balanced on both sides. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for. Copper And Zinc Balanced Equation.
From www.slideserve.com
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379 Copper And Zinc Balanced Equation Balance a chemical equation when given the unbalanced equation. Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Balance a chemical equation when given the unbalanced equation. To balance a chemical. Copper And Zinc Balanced Equation.
From meryes.weebly.com
Balanced chemical equation with state symbols calculator meryes Copper And Zinc Balanced Equation The balanced equation will appear. Explain the roles of subscripts and coefficients in chemical equations. For each element, we check if the number of atoms is balanced on both sides. How do you balance chemical equations step by step? 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. Balance a chemical equation when given the unbalanced. Copper And Zinc Balanced Equation.
From www.slideserve.com
PPT Cells and Voltage PowerPoint Presentation ID5231819 Copper And Zinc Balanced Equation Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two. Copper And Zinc Balanced Equation.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Copper And Zinc Balanced Equation 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. For each element, we check if the number of atoms is balanced on both sides. Balance a chemical equation when given the unbalanced. Copper And Zinc Balanced Equation.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Copper And Zinc Balanced Equation The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Explain the roles of subscripts and coefficients in chemical equations. For each element, we check if the number of atoms is balanced on both sides. Balance a chemical equation when given the unbalanced equation. Balance a chemical equation. Copper And Zinc Balanced Equation.
From www.numerade.com
SOLVED Write the Lewis Dot Structure of Copper Sulfate and Zinc Copper And Zinc Balanced Equation For each element, we check if the number of atoms is balanced on both sides. How do you balance chemical equations step by step? The balanced equation will appear. First, we set all coefficients to 1: Explain the roles of subscripts and coefficients in chemical equations. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where. Copper And Zinc Balanced Equation.
From www.toppr.com
5. Write a balanced chemical equations the following chemical reactions Copper And Zinc Balanced Equation The balanced equation will appear. How do you balance chemical equations step by step? Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. Explain the roles of subscripts and coefficients. Copper And Zinc Balanced Equation.
From www.youtube.com
How to Balance Zn + Cu(NO3)2 = Cu + Zn(NO3)2 YouTube Copper And Zinc Balanced Equation Balance a chemical equation when given the unbalanced equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. How do you balance chemical equations step by step? Explain the roles of subscripts and coefficients in chemical equations. First, we set all coefficients to 1: For each element, we check if the number. Copper And Zinc Balanced Equation.
From chemistryfromscratch.org
V3.9 Copper And Zinc Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. How do you balance chemical equations step by step? Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to.. Copper And Zinc Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + Cu(NO3)2 = Cu + Zn(NO3)2 Copper And Zinc Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. For each element, we check if the number of atoms is balanced on both sides. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. First, we set all coefficients to 1: Balance a chemical equation when given the unbalanced equation. The balanced. Copper And Zinc Balanced Equation.
From www.toppr.com
Write balanced chemical equations for the following word equation Copper And Zinc Balanced Equation How do you balance chemical equations step by step? The balanced equation will appear. Explain the roles of subscripts and coefficients in chemical equations. 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. Explain the roles of subscripts and coefficients in chemical equations. Let us use a double displacement reaction of lead (ii) nitrate and potassium. Copper And Zinc Balanced Equation.
From www.tessshebaylo.com
Write A Balanced Chemical Equation For Each Single Replacement Reaction Copper And Zinc Balanced Equation 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. For each element, we check if the number of atoms is balanced on both sides. How do you balance chemical equations step by step? Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. Explain the roles of subscripts and coefficients in. Copper And Zinc Balanced Equation.
From www.nagwa.com
Question Video Identifying the Correct Chemical Equation for the Copper And Zinc Balanced Equation The balanced equation will appear. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Explain the roles of subscripts and coefficients in chemical equations. For each element, we. Copper And Zinc Balanced Equation.
From www.numerade.com
SOLVED The reaction between zinc and copper sulphate is illustrated Copper And Zinc Balanced Equation Balance a chemical equation when given the unbalanced equation. Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms. Copper And Zinc Balanced Equation.
From www.slideshare.net
Redox= quiz part 1 with answers Copper And Zinc Balanced Equation Balance a chemical equation when given the unbalanced equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balance a chemical equation when given the unbalanced equation. Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. Zn + cuso4 = cu2 + znso4 is a single. Copper And Zinc Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Fe + CuSO4 = Fe2(SO4)3 + Cu Copper And Zinc Balanced Equation The balanced equation will appear. First, we set all coefficients to 1: Let us use a double displacement reaction of lead (ii) nitrate and potassium chromate to. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution) reaction where two moles of solid zinc [zn] and two moles of. Balance a chemical equation when given the unbalanced equation.. Copper And Zinc Balanced Equation.
From slideplayer.com
Chemical Reactions & Equations ppt download Copper And Zinc Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. First, we set all coefficients to 1: How do you balance chemical equations step by step? For each element, we check if the number of atoms is balanced on both sides. Let us use a double displacement. Copper And Zinc Balanced Equation.
From blog.thepipingmart.com
Classifications of Metals Copper vs. Zinc Copper And Zinc Balanced Equation 1 zn + 1 cuso 4 = 1 znso 4 + 1 cu. Explain the roles of subscripts and coefficients in chemical equations. How do you balance chemical equations step by step? The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Balance a chemical equation when. Copper And Zinc Balanced Equation.