Catalysts That Slow Chemical Reactions . Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. In chemistry and biology, a catalyst is a substance the increases the. Production of a gas is a clue that a chemical reaction has occurred. A catalyst lowers the activation energy of a reaction, increasing its rate. Enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a reaction. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. Negative catalysts are substances that have the. It is not consumed by the process. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Tell students that this lesson is. Positive catalysts are substances that have the capacity to speed up a chemical reaction. Also — and this is very important — catalysts affect the forward and.
from slidetodoc.com
Positive catalysts are substances that have the capacity to speed up a chemical reaction. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. In chemistry and biology, a catalyst is a substance the increases the. Tell students that this lesson is. Enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a reaction. It is not consumed by the process. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. A catalyst lowers the activation energy of a reaction, increasing its rate. Production of a gas is a clue that a chemical reaction has occurred. Also — and this is very important — catalysts affect the forward and.
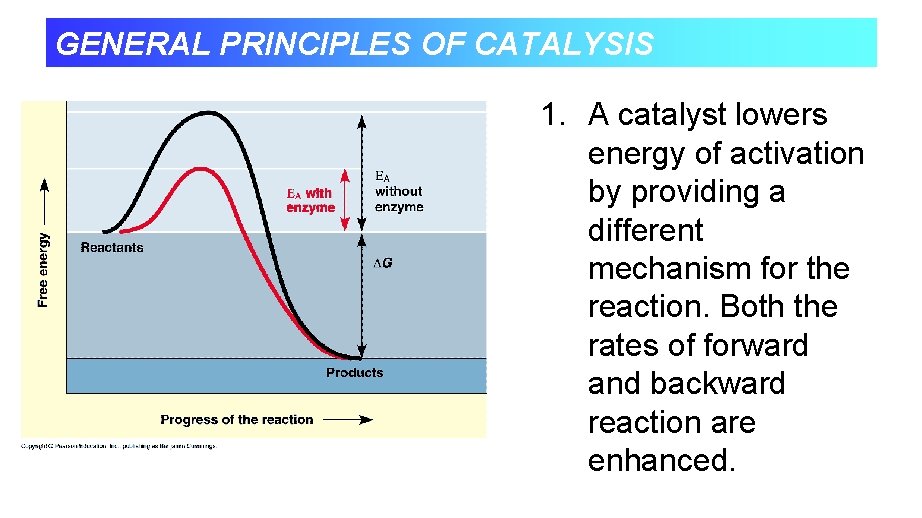
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS
Catalysts That Slow Chemical Reactions In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Negative catalysts are substances that have the. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. A catalyst lowers the activation energy of a reaction, increasing its rate. Tell students that this lesson is. It is not consumed by the process. In chemistry and biology, a catalyst is a substance the increases the. Production of a gas is a clue that a chemical reaction has occurred. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Also — and this is very important — catalysts affect the forward and. Enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a reaction. Positive catalysts are substances that have the capacity to speed up a chemical reaction.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 Catalysts That Slow Chemical Reactions In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. A catalyst lowers the activation energy of a reaction, increasing its rate. Enzymes are. Catalysts That Slow Chemical Reactions.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Catalysts That Slow Chemical Reactions Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Also — and this is very important — catalysts affect the forward and. Enzymes in our bodies are catalysts that speed up. Catalysts That Slow Chemical Reactions.
From www.slideserve.com
PPT Fast and Slow Chemistry PowerPoint Presentation, free download Catalysts That Slow Chemical Reactions Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. Positive catalysts are substances that have the capacity to speed up a chemical reaction. A catalyst lowers the activation energy of a reaction, increasing its rate. It is not consumed by the process. Also — and this is very important — catalysts affect the. Catalysts That Slow Chemical Reactions.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalysts That Slow Chemical Reactions Positive catalysts are substances that have the capacity to speed up a chemical reaction. Tell students that this lesson is. Production of a gas is a clue that a chemical reaction has occurred. It is not consumed by the process. In chemistry and biology, a catalyst is a substance the increases the. A catalyst lowers the activation energy of a. Catalysts That Slow Chemical Reactions.
From slideplayer.com
CHEMICAL REACTIONS. ppt download Catalysts That Slow Chemical Reactions In chemistry and biology, a catalyst is a substance the increases the. Positive catalysts are substances that have the capacity to speed up a chemical reaction. Enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a reaction. Catalysts are substances that speed up a reaction but which are not. Catalysts That Slow Chemical Reactions.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalysts That Slow Chemical Reactions In chemistry and biology, a catalyst is a substance the increases the. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Also — and this is very important — catalysts affect the forward and. Negative catalysts are substances that have the. Catalysts are substances that speed up. Catalysts That Slow Chemical Reactions.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysts That Slow Chemical Reactions Negative catalysts are substances that have the. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Tell students that this lesson is. Production of a gas is a clue that a chemical reaction has occurred. Positive catalysts are substances that have the capacity to speed up a. Catalysts That Slow Chemical Reactions.
From slideplayer.com
Chemical Reactions Types, Energy, and Rates ppt download Catalysts That Slow Chemical Reactions Production of a gas is a clue that a chemical reaction has occurred. Also — and this is very important — catalysts affect the forward and. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under. Catalysts That Slow Chemical Reactions.
From loemotkul.blob.core.windows.net
What Is A Catalyst In A Chemical Reaction at Richard Starr blog Catalysts That Slow Chemical Reactions It is not consumed by the process. A catalyst lowers the activation energy of a reaction, increasing its rate. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to. Catalysts That Slow Chemical Reactions.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalysts That Slow Chemical Reactions A catalyst lowers the activation energy of a reaction, increasing its rate. It is not consumed by the process. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. Also — and this is very important — catalysts affect the forward and. In the absence of enzymatic catalysis, most biochemical reactions are so slow. Catalysts That Slow Chemical Reactions.
From www.slideshare.net
Catalyst Catalysts That Slow Chemical Reactions Production of a gas is a clue that a chemical reaction has occurred. Negative catalysts are substances that have the. Enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a reaction. Also — and this is very important — catalysts affect the forward and. Tell students that this lesson. Catalysts That Slow Chemical Reactions.
From www.pinterest.com.mx
Catalysis Chemistry classroom, Chemistry Catalysts That Slow Chemical Reactions It is not consumed by the process. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Also — and this is very important. Catalysts That Slow Chemical Reactions.
From www.nagwa.com
Question Video Identifying the Reason Why Catalysts Are Used in Catalysts That Slow Chemical Reactions In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Positive catalysts are substances that have the capacity to speed up a chemical reaction. Tell students that this lesson is. In chemistry and biology, a catalyst is a substance the increases the. Enzymes are chemical catalysts that accelerate. Catalysts That Slow Chemical Reactions.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysts That Slow Chemical Reactions Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. Also — and this is very important — catalysts affect the forward and. It is not consumed by the process. In chemistry and biology, a catalyst is a substance the increases the. Catalysts are substances that speed up a reaction but which are not. Catalysts That Slow Chemical Reactions.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalysts That Slow Chemical Reactions It is not consumed by the process. A catalyst lowers the activation energy of a reaction, increasing its rate. In chemistry and biology, a catalyst is a substance the increases the. Also — and this is very important — catalysts affect the forward and. Tell students that this lesson is. Enzymes are chemical catalysts that accelerate chemical reactions at physiological. Catalysts That Slow Chemical Reactions.
From 2012books.lardbucket.org
Catalysis Catalysts That Slow Chemical Reactions In chemistry and biology, a catalyst is a substance the increases the. Negative catalysts are substances that have the. It is not consumed by the process. Positive catalysts are substances that have the capacity to speed up a chemical reaction. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild. Catalysts That Slow Chemical Reactions.
From slideplayer.com
Catalysts Rates of Reactions. ppt download Catalysts That Slow Chemical Reactions It is not consumed by the process. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. In. Catalysts That Slow Chemical Reactions.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalysts That Slow Chemical Reactions In chemistry and biology, a catalyst is a substance the increases the. Also — and this is very important — catalysts affect the forward and. Tell students that this lesson is. Negative catalysts are substances that have the. A catalyst lowers the activation energy of a reaction, increasing its rate. In the absence of enzymatic catalysis, most biochemical reactions are. Catalysts That Slow Chemical Reactions.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts That Slow Chemical Reactions A catalyst lowers the activation energy of a reaction, increasing its rate. Negative catalysts are substances that have the. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to. Catalysts That Slow Chemical Reactions.
From courses.lumenlearning.com
Catalysis Chemistry for Majors Catalysts That Slow Chemical Reactions In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. In chemistry and biology, a catalyst is a substance the increases the. It is not consumed by the process. Production of a gas is a clue that a chemical reaction has occurred. Tell students that this lesson is.. Catalysts That Slow Chemical Reactions.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalysts That Slow Chemical Reactions Negative catalysts are substances that have the. Tell students that this lesson is. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Production of a gas is a clue that a chemical reaction has occurred. In the absence of enzymatic catalysis, most biochemical reactions are. Catalysts That Slow Chemical Reactions.
From slideplayer.com
Chemical Reactions. ppt video online download Catalysts That Slow Chemical Reactions Also — and this is very important — catalysts affect the forward and. Tell students that this lesson is. Negative catalysts are substances that have the. Production of a gas is a clue that a chemical reaction has occurred. In chemistry and biology, a catalyst is a substance the increases the. A catalyst lowers the activation energy of a reaction,. Catalysts That Slow Chemical Reactions.
From www.slideserve.com
PPT Basic Chemistry Concepts PowerPoint Presentation, free download Catalysts That Slow Chemical Reactions Also — and this is very important — catalysts affect the forward and. Production of a gas is a clue that a chemical reaction has occurred. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Tell students that this lesson is. Positive catalysts are substances. Catalysts That Slow Chemical Reactions.
From slidetodoc.com
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS Catalysts That Slow Chemical Reactions In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Also — and this is very important — catalysts affect the forward and. Negative catalysts are substances that have the. Tell students that this lesson is. Catalysts are substances that speed up a reaction but which are not. Catalysts That Slow Chemical Reactions.
From loemotkul.blob.core.windows.net
What Is A Catalyst In A Chemical Reaction at Richard Starr blog Catalysts That Slow Chemical Reactions It is not consumed by the process. Also — and this is very important — catalysts affect the forward and. Tell students that this lesson is. Positive catalysts are substances that have the capacity to speed up a chemical reaction. Enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start. Catalysts That Slow Chemical Reactions.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalysts That Slow Chemical Reactions Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Production of a gas is a clue that a chemical reaction has occurred. A catalyst lowers the activation energy of a reaction, increasing its rate. In chemistry and biology, a catalyst is a substance the increases. Catalysts That Slow Chemical Reactions.
From slideplayer.com
Chapter 12 Chemical Reactions. ppt download Catalysts That Slow Chemical Reactions Negative catalysts are substances that have the. Also — and this is very important — catalysts affect the forward and. In chemistry and biology, a catalyst is a substance the increases the. Positive catalysts are substances that have the capacity to speed up a chemical reaction. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they. Catalysts That Slow Chemical Reactions.
From slideplayer.com
CHEMISTRY 2 REVISION Periodic Table, Chemical Reaction Rate, Fuels Catalysts That Slow Chemical Reactions A catalyst lowers the activation energy of a reaction, increasing its rate. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a reaction. Tell students that this. Catalysts That Slow Chemical Reactions.
From slideplayer.com
Chapter 6 4 Chemical Reactions ppt download Catalysts That Slow Chemical Reactions Tell students that this lesson is. Enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a reaction. In chemistry and biology, a catalyst is a substance the increases the. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild. Catalysts That Slow Chemical Reactions.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalysts That Slow Chemical Reactions In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Negative catalysts are substances that have the. Positive catalysts are substances that have the capacity to speed up a chemical reaction. A catalyst lowers the activation energy of a reaction, increasing its rate. Production of a gas is. Catalysts That Slow Chemical Reactions.
From www.youtube.com
Reaction Mechanisms and Rate Laws fast and slow first step YouTube Catalysts That Slow Chemical Reactions In chemistry and biology, a catalyst is a substance the increases the. In the absence of enzymatic catalysis, most biochemical reactions are so slow that they would not occur under the mild conditions of. Tell students that this lesson is. It is not consumed by the process. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. Catalysts That Slow Chemical Reactions.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalysts That Slow Chemical Reactions Enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a reaction. Negative catalysts are substances that have the. Tell students that this lesson is. It is not consumed by the process. Catalysts are substances that speed up a reaction but which are not consumed by it and do not. Catalysts That Slow Chemical Reactions.
From www.waca.msf.org
Catalyst, Catalyst Catalysts That Slow Chemical Reactions Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. In chemistry and biology, a catalyst is a substance the increases the. Also — and this is very important — catalysts affect the forward and. A catalyst lowers the activation energy of a reaction, increasing its. Catalysts That Slow Chemical Reactions.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalysts That Slow Chemical Reactions Tell students that this lesson is. Negative catalysts are substances that have the. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. In chemistry and biology, a catalyst is a substance the increases the. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts are substances that speed up a. Catalysts That Slow Chemical Reactions.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalysts That Slow Chemical Reactions In chemistry and biology, a catalyst is a substance the increases the. Tell students that this lesson is. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Production of. Catalysts That Slow Chemical Reactions.