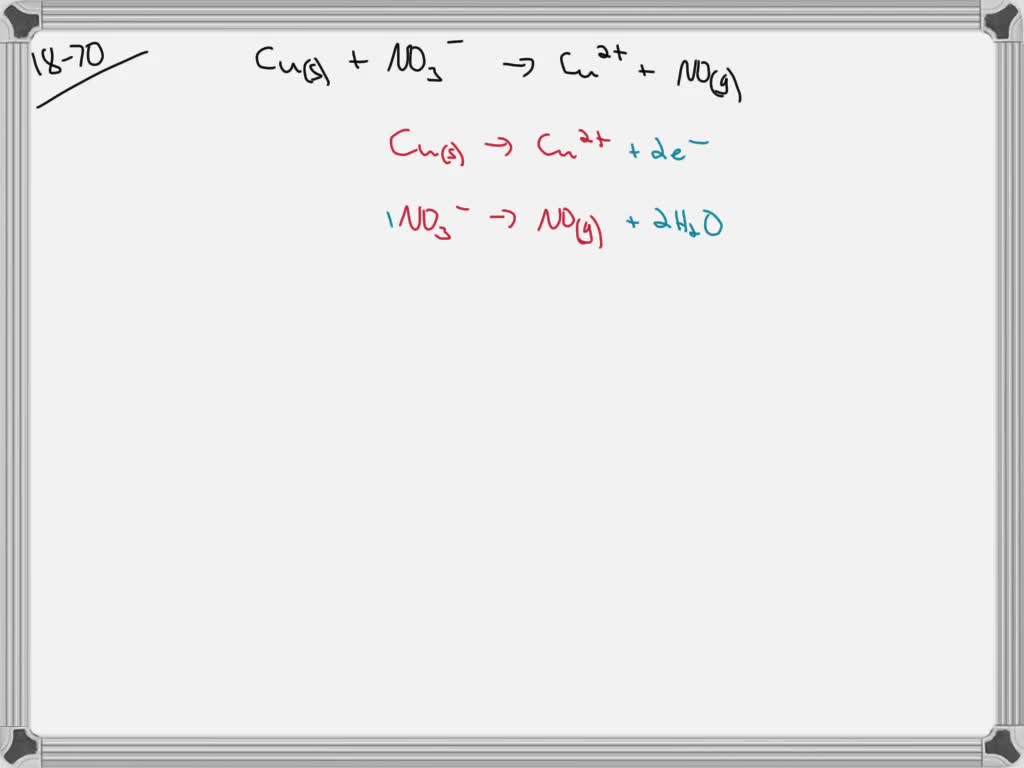Dilute Nitric Acid Formula . This yields the most general form of the dilution equation: See examples of nitric acid reactions. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. Write the formula for calculating dilution. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means. Use the calculator to find the mass, volume and. When ordered from a chemical supply company, its molarity is \(16 \: Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid depending on the concentration and temperature. It is a strong monobasic acid and a powerful oxidizing agent. How to prepare 10 percent nitric acid solution? Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock solutions. Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid.
from www.numerade.com
Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. When ordered from a chemical supply company, its molarity is \(16 \: See examples of nitric acid reactions. Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock solutions. How to prepare 10 percent nitric acid solution? Write the formula for calculating dilution. Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid depending on the concentration and temperature. This yields the most general form of the dilution equation: Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means.
SOLVEDWrite a balanced equation for the reaction of a copper penny in
Dilute Nitric Acid Formula Write the formula for calculating dilution. Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid depending on the concentration and temperature. Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. Write the formula for calculating dilution. When ordered from a chemical supply company, its molarity is \(16 \: This yields the most general form of the dilution equation: How to prepare 10 percent nitric acid solution? Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock solutions. Use the calculator to find the mass, volume and. It is a strong monobasic acid and a powerful oxidizing agent. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. See examples of nitric acid reactions.
From www.numerade.com
SOLVED The equation for the reaction of copper and dilute nitric acid Dilute Nitric Acid Formula Write the formula for calculating dilution. It is a strong monobasic acid and a powerful oxidizing agent. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means. See examples of nitric acid reactions. This yields the most general form of the dilution equation: Nitric acid \(\left( \ce{hno_3}. Dilute Nitric Acid Formula.
From www.toppr.com
Write the balanced chemical equation when zinc reacts with dilute Dilute Nitric Acid Formula Write the formula for calculating dilution. How to prepare 10 percent nitric acid solution? Use the calculator to find the mass, volume and. Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock solutions. It is a strong monobasic acid and a powerful oxidizing agent. When ordered from a chemical supply company, its molarity. Dilute Nitric Acid Formula.
From www.numerade.com
SOLVED The equation for the reaction of copper and dilute nitric acid Dilute Nitric Acid Formula How to prepare 10 percent nitric acid solution? Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid depending on the concentration and temperature. Write the formula for calculating dilution. Use the calculator. Dilute Nitric Acid Formula.
From www.numerade.com
SOLVED When copper(II) oxide reacts with dilute nitric acid, copper(II Dilute Nitric Acid Formula See examples of nitric acid reactions. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid depending on the concentration and temperature. Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid.. Dilute Nitric Acid Formula.
From www.youtube.com
When zinc reacts with very dilute nitric acid it produces YouTube Dilute Nitric Acid Formula See examples of nitric acid reactions. It is a strong monobasic acid and a powerful oxidizing agent. Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. How to prepare 10 percent nitric acid solution? Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid. Dilute Nitric Acid Formula.
From www.nagwa.com
Lesson Video Properties of Nitric Acid Nagwa Dilute Nitric Acid Formula Write the formula for calculating dilution. Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid depending on the concentration and temperature. It is a strong monobasic acid and a powerful oxidizing agent. How to prepare 10 percent nitric acid solution? This yields the most general. Dilute Nitric Acid Formula.
From www.chegg.com
Solved Iron solid can react with dilute nitric acid to form Dilute Nitric Acid Formula See examples of nitric acid reactions. It is a strong monobasic acid and a powerful oxidizing agent. Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock solutions. Write the. Dilute Nitric Acid Formula.
From www.numerade.com
SOLVEDWrite a balanced equation for the reaction of a copper penny in Dilute Nitric Acid Formula Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. When ordered from a chemical supply company, its molarity is \(16 \: Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock solutions. Write the formula for calculating dilution. It is a strong monobasic acid and a powerful oxidizing agent. This. Dilute Nitric Acid Formula.
From www.numerade.com
SOLVEDBalance the following equation for the reaction of magnesium Dilute Nitric Acid Formula This yields the most general form of the dilution equation: \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means. It is a strong monobasic acid and a powerful oxidizing agent. When ordered from a chemical supply company, its molarity is \(16 \: Nitric acid \(\left( \ce{hno_3}. Dilute Nitric Acid Formula.
From www.toppr.com
Write the balanced chemical equation when zinc reacts with dilute Dilute Nitric Acid Formula Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock solutions. See examples of nitric acid reactions. Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid. Dilute Nitric Acid Formula.
From ar.inspiredpencil.com
Nitric Acid Structure Dilute Nitric Acid Formula Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid depending on the concentration and temperature. Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a. Dilute Nitric Acid Formula.
From exopkbipv.blob.core.windows.net
Dilution Equation Example Chemistry at Wanda King blog Dilute Nitric Acid Formula It is a strong monobasic acid and a powerful oxidizing agent. Use the calculator to find the mass, volume and. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means. When ordered from a chemical supply company, its molarity is \(16 \: Write the formula for calculating. Dilute Nitric Acid Formula.
From www.youtube.com
Write fully balanced equation for the reaction of dilute nitric acid Dilute Nitric Acid Formula Write the formula for calculating dilution. Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock solutions. Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid depending on the concentration and temperature. \[c_1v_1=c_2v_2\] to summarize, c stands for the. Dilute Nitric Acid Formula.
From www.youtube.com
sodium carbonate and nitric acid total ionic equation YouTube Dilute Nitric Acid Formula This yields the most general form of the dilution equation: Use the calculator to find the mass, volume and. It is a strong monobasic acid and a powerful oxidizing agent. When ordered from a chemical supply company, its molarity is \(16 \: How to prepare 10 percent nitric acid solution? Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive. Dilute Nitric Acid Formula.
From www.coursehero.com
[Solved] . The equation for the reaction of copper and dilute nitric Dilute Nitric Acid Formula \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. It is a strong monobasic acid and a powerful oxidizing agent. Write the formula for calculating dilution. Learn about the different types of reactions. Dilute Nitric Acid Formula.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Dilute Nitric Acid Formula Write the formula for calculating dilution. See examples of nitric acid reactions. Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. Use the calculator to find the mass, volume and. Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid depending on the concentration. Dilute Nitric Acid Formula.
From edurev.in
The reaction of zinc with dilute and concentrated nitric acid Dilute Nitric Acid Formula Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. See examples of nitric acid reactions. It is a strong monobasic acid and a powerful oxidizing agent. How to prepare 10 percent nitric acid solution? \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means. This. Dilute Nitric Acid Formula.
From www.youtube.com
How to Balance Pb + HNO3 = Pb(NO3)2 + NO2 + H2O (Note Dilute Nitric Dilute Nitric Acid Formula See examples of nitric acid reactions. When ordered from a chemical supply company, its molarity is \(16 \: \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means. Write the formula for calculating dilution. Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from. Dilute Nitric Acid Formula.
From www.dreamstime.com
Drawn Molecule and Formula of Nitric Acid Stock Vector Illustration Dilute Nitric Acid Formula See examples of nitric acid reactions. Use the calculator to find the mass, volume and. How to prepare 10 percent nitric acid solution? It is a strong monobasic acid and a powerful oxidizing agent. Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. Learn about the different types of reactions of nitric acid, a strong acid that can. Dilute Nitric Acid Formula.
From www.numerade.com
SOLVEDWrite a balanced net ionic equation for each of the following Dilute Nitric Acid Formula Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. See examples of nitric acid reactions. How to prepare 10 percent nitric acid solution? \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means. Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3). Dilute Nitric Acid Formula.
From pixels.com
Nitric Acid Molecule Photograph by Molekuul/science Photo Library Pixels Dilute Nitric Acid Formula Use the calculator to find the mass, volume and. See examples of nitric acid reactions. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means. Write the formula for calculating dilution. Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock. Dilute Nitric Acid Formula.
From www.dreamstime.com
Nitric acid molecule stock illustration. Illustration of aqua 179647247 Dilute Nitric Acid Formula How to prepare 10 percent nitric acid solution? It is a strong monobasic acid and a powerful oxidizing agent. Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. Use the calculator to find the mass, volume and. This yields the most general form of the dilution equation: Learn how to prepare a 0.1 mol/l solution of nitric acid. Dilute Nitric Acid Formula.
From klafwpnrs.blob.core.windows.net
Dilution Formula Explanation at John Schell blog Dilute Nitric Acid Formula Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. When ordered from a chemical supply company, its molarity is \(16 \: This yields the most general form of the dilution equation: Write the formula for calculating dilution. See examples of nitric acid reactions. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. How to. Dilute Nitric Acid Formula.
From exopkbipv.blob.core.windows.net
Dilution Equation Example Chemistry at Wanda King blog Dilute Nitric Acid Formula Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock solutions. It is a strong monobasic acid and a powerful oxidizing agent. Use the calculator to find the mass, volume and. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means.. Dilute Nitric Acid Formula.
From www.dreamstime.com
Nitric acid molecule stock illustration. Illustration of formula Dilute Nitric Acid Formula How to prepare 10 percent nitric acid solution? Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid depending on the concentration and temperature. Write the formula for calculating dilution. This yields the most general. Dilute Nitric Acid Formula.
From www.chemicals.co.uk
What Is Nitric Acid? The Chemistry Blog Dilute Nitric Acid Formula Write the formula for calculating dilution. Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid depending on the concentration and temperature. How to prepare 10 percent nitric acid solution? It is a strong monobasic acid and a powerful oxidizing agent. See examples of nitric acid. Dilute Nitric Acid Formula.
From www.gbu-taganskij.ru
Nitric Acid Or Hno3 Molecule Skeletal Formula Vector Image, 44 OFF Dilute Nitric Acid Formula It is a strong monobasic acid and a powerful oxidizing agent. Use the calculator to find the mass, volume and. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means. Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. Learn about the different types of. Dilute Nitric Acid Formula.
From www.quanswer.com
Write chemical equation of reaction between dilute nitric acid and Dilute Nitric Acid Formula Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock solutions. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. See examples of nitric acid reactions. Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid. Dilute Nitric Acid Formula.
From www.vectorstock.com
Nitric acid hno3 strong mineral acid molecule Vector Image Dilute Nitric Acid Formula It is a strong monobasic acid and a powerful oxidizing agent. This yields the most general form of the dilution equation: Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. Use the calculator to find the mass, volume and. How to prepare 10 percent nitric acid solution? Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and. Dilute Nitric Acid Formula.
From www.youtube.com
How to Write the Net Ionic Equation for HNO3 (Dilute) + Mg = Mg(NO3)2 Dilute Nitric Acid Formula When ordered from a chemical supply company, its molarity is \(16 \: How to prepare 10 percent nitric acid solution? Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. See examples of nitric acid reactions. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of. Dilute Nitric Acid Formula.
From www.numerade.com
The equation for the reaction between calcium carbonate and dilute Dilute Nitric Acid Formula Use the calculator to find the mass, volume and. Write the formula for calculating dilution. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. See examples of nitric acid reactions. This yields the most general form of the dilution equation: It is a strong monobasic acid and a powerful oxidizing agent. How to prepare 10 percent. Dilute Nitric Acid Formula.
From byjus.com
Copper reacts with dilute nitric acid and forms copper nitrate, nitric Dilute Nitric Acid Formula Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock solutions. Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. How to prepare 10 percent nitric acid solution? It is a strong monobasic acid and a powerful oxidizing agent. Learn about the different types of reactions of nitric acid, a strong acid. Dilute Nitric Acid Formula.
From mavink.com
Nitric Acid Lewis Structure Dilute Nitric Acid Formula Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. It is a strong monobasic acid and a powerful oxidizing agent. How to prepare 10 percent nitric acid solution? Learn about the different types of reactions of nitric acid, a strong acid that can behave as an oxidizing acid or a typical acid depending on the concentration. Dilute Nitric Acid Formula.
From stock.adobe.com
Nitric acid molecule model illustration HNO3 Stock Vector Adobe Stock Dilute Nitric Acid Formula When ordered from a chemical supply company, its molarity is \(16 \: See examples of nitric acid reactions. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock solutions. How to prepare 10 percent nitric acid solution? Learn about the different. Dilute Nitric Acid Formula.
From www.slideserve.com
PPT A.In dilute nitric acid, HNO3, copper metal dissolves according Dilute Nitric Acid Formula Nitric acid \(\left( \ce{hno_3} \right)\) is a powerful and corrosive acid. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. See examples of nitric acid reactions. Learn how to prepare a 0.1 mol/l solution of nitric acid (hno3) from solid or stock solutions. Learn about the different types of reactions of nitric acid, a strong acid. Dilute Nitric Acid Formula.