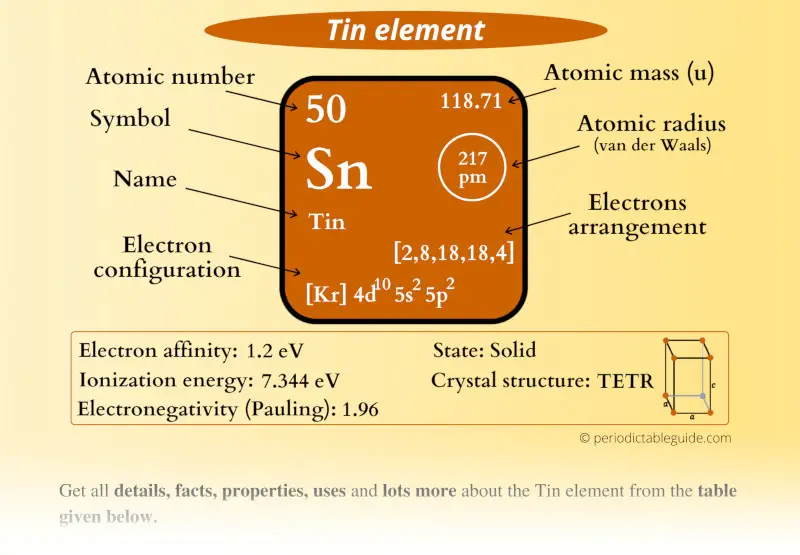
Tin Electron Charge . When the tin atom loses two electrons from the 5p orbital it becomes sn 2+ ion and when it loses two more electrons from the 5s orbital it becomes sn 4+ ion. Tin can have a +2 or +4 depending on how it reacts. Tin (sn) has an atomic mass of 50. This configuration provides insight into its chemical behavior, primarily its ability to form compounds. Since the 1s orbital can hold only two electrons the next two will enter the 2s orbital. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. What is the charge of sn? Tin has the electron configuration [kr] 4d¹⁰ 5s² 5p². Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds with its. To write the electron configuration for tin, the first two electrons enter the 1s orbital. To form the sn 4+ ion, tin loses all four electrons in the 5s. Melting point the temperature at which the. Electron configuration the arrangements of electrons above the last (closed shell) noble gas.
from periodictableguide.com
When the tin atom loses two electrons from the 5p orbital it becomes sn 2+ ion and when it loses two more electrons from the 5s orbital it becomes sn 4+ ion. To form the sn 4+ ion, tin loses all four electrons in the 5s. Melting point the temperature at which the. To write the electron configuration for tin, the first two electrons enter the 1s orbital. Tin has the electron configuration [kr] 4d¹⁰ 5s² 5p². What is the charge of sn? To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. This configuration provides insight into its chemical behavior, primarily its ability to form compounds. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Tin (sn) has an atomic mass of 50.
Tin (Sn) Periodic Table (Element Information & More)
Tin Electron Charge Melting point the temperature at which the. What is the charge of sn? Tin (sn) has an atomic mass of 50. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. When the tin atom loses two electrons from the 5p orbital it becomes sn 2+ ion and when it loses two more electrons from the 5s orbital it becomes sn 4+ ion. Tin has the electron configuration [kr] 4d¹⁰ 5s² 5p². To form the sn 4+ ion, tin loses all four electrons in the 5s. Tin can have a +2 or +4 depending on how it reacts. This configuration provides insight into its chemical behavior, primarily its ability to form compounds. Since the 1s orbital can hold only two electrons the next two will enter the 2s orbital. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds with its. Melting point the temperature at which the. To write the electron configuration for tin, the first two electrons enter the 1s orbital.
From www.youtube.com
How to Find the Valence Electrons for Tin (Sn) YouTube Tin Electron Charge Electron configuration the arrangements of electrons above the last (closed shell) noble gas. To form the sn 4+ ion, tin loses all four electrons in the 5s. Melting point the temperature at which the. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. Tin has a ground state electron configuration of 1s 2. Tin Electron Charge.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Electron Charge Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds with its. Melting point the temperature at which the. Tin can have a +2 or +4 depending on how it reacts. Find out. Tin Electron Charge.
From www.alamy.com
Tin (Sn). Diagram of the nuclear composition and electron configuration Tin Electron Charge Electron configuration the arrangements of electrons above the last (closed shell) noble gas. To write the electron configuration for tin, the first two electrons enter the 1s orbital. This configuration provides insight into its chemical behavior, primarily its ability to form compounds. Melting point the temperature at which the. Tin can have a +2 or +4 depending on how it. Tin Electron Charge.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Electron Charge Electron configuration the arrangements of electrons above the last (closed shell) noble gas. This configuration provides insight into its chemical behavior, primarily its ability to form compounds. Tin can have a +2 or +4 depending on how it reacts. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. When the tin atom loses two electrons. Tin Electron Charge.
From ar.inspiredpencil.com
Tin Atomic Structure Tin Electron Charge Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Since the 1s orbital can hold only two electrons the next two will enter the 2s orbital. When the tin atom loses two electrons from the 5p orbital it becomes sn 2+ ion and when it loses two more electrons from the 5s orbital it becomes. Tin Electron Charge.
From commons.wikimedia.org
FileElectron shell 050 tin.png Tin Electron Charge Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Since the 1s orbital can hold only two electrons the next two will enter the 2s orbital. Tin can have a +2 or +4 depending on how it reacts. To form the sn 4+ ion, tin loses all four electrons in the 5s. When the tin atom. Tin Electron Charge.
From www.youtube.com
Sn^2+,Sn^4+ and Sn (Tin and Tin Ions) Electron ConfigurationCrash Tin Electron Charge Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds with its. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. To form the sn 4+ ion, tin loses. Tin Electron Charge.
From periodictableguide.com
Tin (Sn) Periodic Table (Element Information & More) Tin Electron Charge Tin can have a +2 or +4 depending on how it reacts. Tin has the electron configuration [kr] 4d¹⁰ 5s² 5p². Since the 1s orbital can hold only two electrons the next two will enter the 2s orbital. This configuration provides insight into its chemical behavior, primarily its ability to form compounds. To write the electron configuration for tin, the. Tin Electron Charge.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Electron Charge Electron configuration the arrangements of electrons above the last (closed shell) noble gas. What is the charge of sn? Since the 1s orbital can hold only two electrons the next two will enter the 2s orbital. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6. Tin Electron Charge.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Tin Have? Tin Electron Charge To form the sn 4+ ion, tin loses all four electrons in the 5s. Tin has the electron configuration [kr] 4d¹⁰ 5s² 5p². Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds. Tin Electron Charge.
From www.nuclear-power.com
Tin Electron Affinity Electronegativity Ionization Energy of Tin Tin Electron Charge Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Tin has the electron configuration [kr] 4d¹⁰ 5s² 5p². When the tin atom loses two electrons from the 5p orbital it becomes sn 2+ ion and when it loses two more electrons from the 5s orbital it becomes sn 4+ ion. This configuration provides insight into its. Tin Electron Charge.
From www.alamy.com
Symbol and electron diagram for Tin illustration Stock Vector Image Tin Electron Charge Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. What is the charge of sn? Since the 1s orbital can hold only two electrons the next two will enter the 2s orbital. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. Tin (sn) has an atomic mass of. Tin Electron Charge.
From www.youtube.com
Electron Configuration of Tin Sn Lesson YouTube Tin Electron Charge Tin (sn) has an atomic mass of 50. To write the electron configuration for tin, the first two electrons enter the 1s orbital. Tin has the electron configuration [kr] 4d¹⁰ 5s² 5p². Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. To form sn 2+, tin loses two electrons from its 5s 2 or 5p. Tin Electron Charge.
From sciencenotes.org
Element Charges Chart How to Know the Charge of an Atom Tin Electron Charge Since the 1s orbital can hold only two electrons the next two will enter the 2s orbital. This configuration provides insight into its chemical behavior, primarily its ability to form compounds. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Melting point the temperature at which the. Tin has the electron configuration [kr] 4d¹⁰ 5s² 5p².. Tin Electron Charge.
From valenceelectrons.com
How to find the Protons, Neutrons and Electrons for Tin? Tin Electron Charge Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds with its. When the tin atom loses two electrons from the 5p orbital it becomes sn 2+ ion and when it loses two. Tin Electron Charge.
From depositphotos.com
Symbol and electron diagram for Tin Stock Vector by ©blueringmedia 83746444 Tin Electron Charge This configuration provides insight into its chemical behavior, primarily its ability to form compounds. Tin (sn) has an atomic mass of 50. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds with. Tin Electron Charge.
From www.colourbox.com
Symbol and electron diagram for Tin illustration Stock Vector Colourbox Tin Electron Charge Melting point the temperature at which the. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds with its. When the tin atom loses two electrons from the 5p orbital it becomes sn. Tin Electron Charge.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Compare Sodium vs Tin Periodic Table Element Comparison Compare Tin Electron Charge To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. To write the electron configuration for tin, the first two electrons enter the 1s orbital. What is the charge of sn? This configuration provides insight into its chemical behavior, primarily its ability to form compounds. Tin (sn) has an atomic mass of 50. Find. Tin Electron Charge.
From ar.inspiredpencil.com
Tin Atomic Structure Tin Electron Charge Tin can have a +2 or +4 depending on how it reacts. To form the sn 4+ ion, tin loses all four electrons in the 5s. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Tin (sn) has an atomic mass of 50. To write the electron configuration for tin, the first two electrons enter the. Tin Electron Charge.
From www.chemistrylearner.com
Tin Definition, Facts, Symbol, Discovery, Property, Uses Tin Electron Charge Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds with its. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. Tin (sn) has an atomic. Tin Electron Charge.
From depositphotos.com
Tin Electron Configuration Its Properties Vector Illustration Stock Tin Electron Charge Tin has the electron configuration [kr] 4d¹⁰ 5s² 5p². To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. Tin can have a +2 or +4 depending on how it reacts. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Tin has a ground state electron configuration of 1s. Tin Electron Charge.
From www.britannica.com
Tin Definition, Properties, Uses, & Facts Britannica Tin Electron Charge Tin can have a +2 or +4 depending on how it reacts. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds with its. What is the charge of sn? Find out about. Tin Electron Charge.
From valenceelectrons.com
Tin(Sn) electron configuration and orbital diagram Tin Electron Charge Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds with its. When the tin atom loses two electrons from. Tin Electron Charge.
From www.schoolmykids.com
Tin (Sn) Element Information, Facts, Properties, Uses Periodic Tin Electron Charge To form the sn 4+ ion, tin loses all four electrons in the 5s. When the tin atom loses two electrons from the 5p orbital it becomes sn 2+ ion and when it loses two more electrons from the 5s orbital it becomes sn 4+ ion. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. To. Tin Electron Charge.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Electron Charge Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Tin has the electron configuration [kr] 4d¹⁰ 5s² 5p². Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds with. Tin Electron Charge.
From www.shutterstock.com
Tin Electron Configuration Propertiesvector Illustration Stock Vector Tin Electron Charge Electron configuration the arrangements of electrons above the last (closed shell) noble gas. This configuration provides insight into its chemical behavior, primarily its ability to form compounds. To form the sn 4+ ion, tin loses all four electrons in the 5s. When the tin atom loses two electrons from the 5p orbital it becomes sn 2+ ion and when it. Tin Electron Charge.
From animalia-life.club
Periodic Table Electron Charges Tin Electron Charge To form the sn 4+ ion, tin loses all four electrons in the 5s. Since the 1s orbital can hold only two electrons the next two will enter the 2s orbital. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Melting point the temperature at which the. Tin has a ground state electron configuration of. Tin Electron Charge.
From periodictable.me
What are the Difference Between Charge and Electron? Tin Electron Charge Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds with its. This configuration provides insight into its chemical behavior, primarily its ability to form compounds. Electron configuration the arrangements of electrons above. Tin Electron Charge.
From www.vectorstock.com
Symbol and electron diagram for tin Royalty Free Vector Tin Electron Charge Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Tin (sn) has an atomic mass of 50. To write the electron configuration for tin, the first two electrons enter the 1s orbital. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. To form the sn 4+ ion, tin loses all four. Tin Electron Charge.
From ar.inspiredpencil.com
Tin Atomic Structure Tin Electron Charge Tin has the electron configuration [kr] 4d¹⁰ 5s² 5p². What is the charge of sn? Tin (sn) has an atomic mass of 50. To write the electron configuration for tin, the first two electrons enter the 1s orbital. Tin can have a +2 or +4 depending on how it reacts. This configuration provides insight into its chemical behavior, primarily its. Tin Electron Charge.
From www.alamy.com
3d render of atom structure of tin isolated over white background Tin Electron Charge To form the sn 4+ ion, tin loses all four electrons in the 5s. Tin (sn) has an atomic mass of 50. When the tin atom loses two electrons from the 5p orbital it becomes sn 2+ ion and when it loses two more electrons from the 5s orbital it becomes sn 4+ ion. Tin has the electron configuration [kr]. Tin Electron Charge.
From www.sciencephoto.com
Tin, atomic structure Stock Image C018/3731 Science Photo Library Tin Electron Charge Melting point the temperature at which the. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii) compounds with its. To write the electron configuration for tin, the first two electrons enter the 1s. Tin Electron Charge.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Electron Charge To form the sn 4+ ion, tin loses all four electrons in the 5s. Tin can have a +2 or +4 depending on how it reacts. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Tin has the electron configuration [kr] 4d¹⁰ 5s² 5p². To form sn 2+, tin loses two electrons from its 5s. Tin Electron Charge.
From www.alamy.com
Sn Tin, Periodic Table of the Elements, Shell Structure of Tin Tin Electron Charge When the tin atom loses two electrons from the 5p orbital it becomes sn 2+ ion and when it loses two more electrons from the 5s orbital it becomes sn 4+ ion. This configuration provides insight into its chemical behavior, primarily its ability to form compounds. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6. Tin Electron Charge.
From www.dreamstime.com
Atom of Tin with Detailed Core and Its 50 Electrons with Atoms Stock Tin Electron Charge Tin (sn) has an atomic mass of 50. To write the electron configuration for tin, the first two electrons enter the 1s orbital. What is the charge of sn? Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and. Tin Electron Charge.