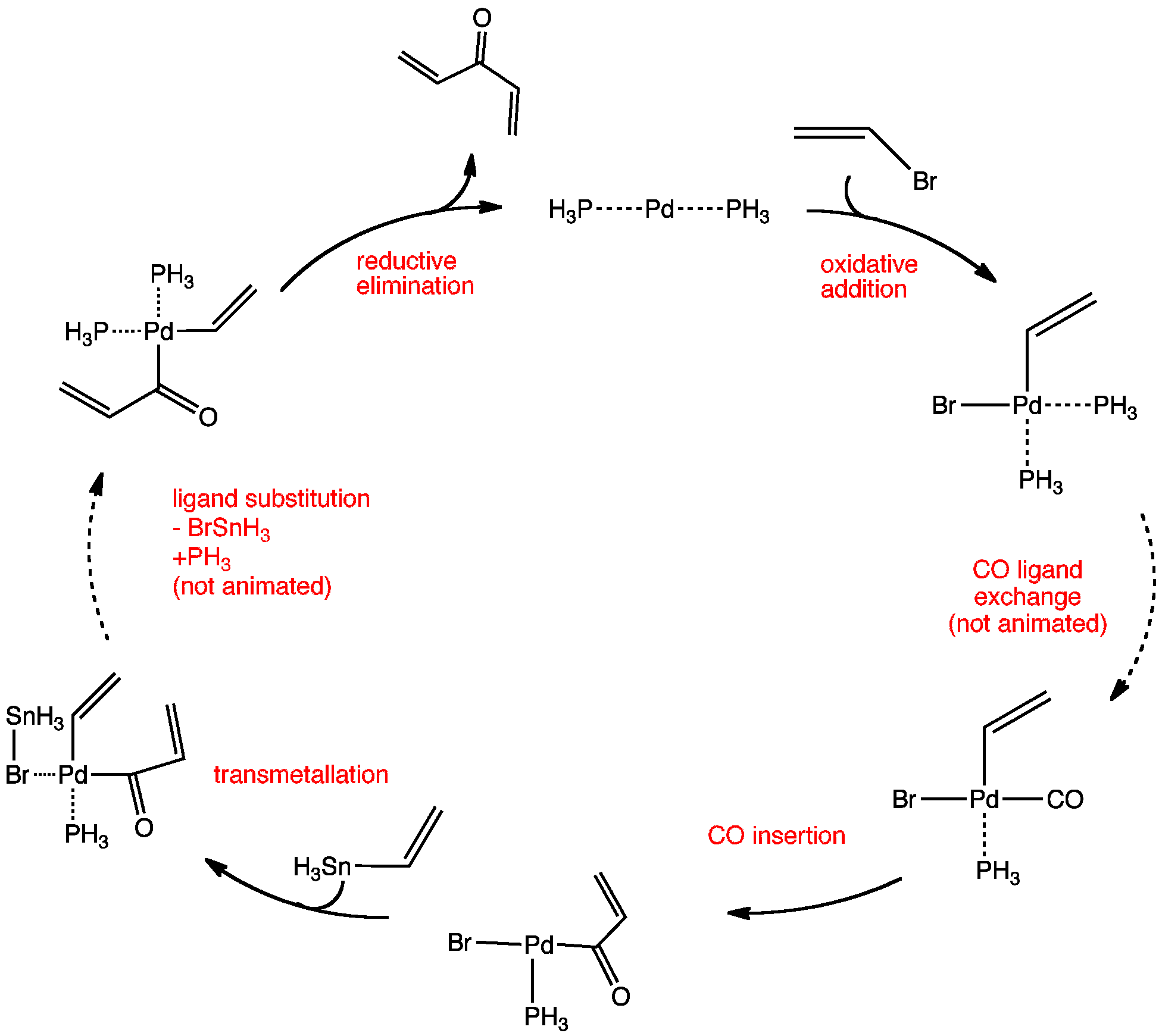
Conditions For Coupling Reaction . One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. One simple example of the coupling of reaction is the decomposition of calcium carbonate: Typically, cross coupling reactions are run in organic conditions; Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; If the temperature is raised above 837 ºc, this reaction becomes. Mechanism of the suzuki coupling.
from www.chemtube3d.com
\[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. Typically, cross coupling reactions are run in organic conditions; One simple example of the coupling of reaction is the decomposition of calcium carbonate: Mechanism of the suzuki coupling. If the temperature is raised above 837 ºc, this reaction becomes. Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction.
Organopalladium Chemistry The Carbonylative KosugiMigitaStille
Conditions For Coupling Reaction One simple example of the coupling of reaction is the decomposition of calcium carbonate: One simple example of the coupling of reaction is the decomposition of calcium carbonate: Typically, cross coupling reactions are run in organic conditions; If the temperature is raised above 837 ºc, this reaction becomes. One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. Mechanism of the suzuki coupling.
From chemistry-reaction.com
Stille Coupling Reaction Mechanism with Application Conditions For Coupling Reaction Typically, cross coupling reactions are run in organic conditions; Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. One simple example of the coupling of reaction is the decomposition of calcium carbonate: A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together. Conditions For Coupling Reaction.
From www.researchgate.net
Representative examples for coupling reactions Isolated Conditions For Coupling Reaction One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. If the temperature is raised above 837 ºc, this reaction. Conditions For Coupling Reaction.
From www.researchgate.net
Optimization of reaction conditions in Heck coupling reaction of Conditions For Coupling Reaction Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. If the temperature is raised above 837 ºc, this reaction becomes. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. Mechanism of the suzuki coupling.. Conditions For Coupling Reaction.
From www.science.org
Nickelcatalyzed hydrogenative coupling of nitriles and amines for Conditions For Coupling Reaction Mechanism of the suzuki coupling. Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. If the temperature is raised above 837 ºc, this reaction becomes. One simple example of the coupling of reaction is the decomposition of calcium carbonate: A coupling reaction in organic chemistry is a general term for various. Conditions For Coupling Reaction.
From www.researchgate.net
Reaction condition optimization for Suzuki coupling of thiazole Conditions For Coupling Reaction One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. Mechanism of the suzuki coupling. One simple example of the coupling of reaction is the decomposition of calcium carbonate: If the temperature is raised above 837 ºc, this reaction becomes. Typically, cross coupling reactions are run. Conditions For Coupling Reaction.
From www.researchgate.net
Substrate scope for Suzuki−Miyaura coupling reaction using the Conditions For Coupling Reaction \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. One simple example of the coupling of reaction is the. Conditions For Coupling Reaction.
From chemistry-reaction.com
Suzuki crosscoupling Reaction Examples Mechanism Application Conditions For Coupling Reaction Mechanism of the suzuki coupling. One simple example of the coupling of reaction is the decomposition of calcium carbonate: One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the. Conditions For Coupling Reaction.
From www.researchgate.net
Screening of conditions for the crosscoupling reaction a Download Table Conditions For Coupling Reaction Typically, cross coupling reactions are run in organic conditions; If the temperature is raised above 837 ºc, this reaction becomes. One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. One simple example of the coupling of reaction is the decomposition of calcium carbonate: \[caco_{3(s)} \rightleftharpoons. Conditions For Coupling Reaction.
From www.chemtube3d.com
Organopalladium Chemistry The Carbonylative KosugiMigitaStille Conditions For Coupling Reaction \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; If the temperature is raised above 837 ºc, this reaction becomes. Mechanism of the suzuki coupling. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. One simple example of the coupling of reaction is the decomposition of. Conditions For Coupling Reaction.
From www.researchgate.net
Optimisation of coupling reaction conditions All reactions were carried Conditions For Coupling Reaction One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. If the temperature is raised above 837 ºc, this reaction. Conditions For Coupling Reaction.
From www.researchgate.net
Survey of the Reaction Condition for CuCatalyzed Coupling Reaction a Conditions For Coupling Reaction One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. If the temperature is raised above 837 ºc, this reaction becomes. Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. Typically, cross coupling reactions are run in. Conditions For Coupling Reaction.
From www.researchgate.net
Mechanism of coupling reaction. Download Scientific Diagram Conditions For Coupling Reaction Mechanism of the suzuki coupling. One simple example of the coupling of reaction is the decomposition of calcium carbonate: Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. Typically, cross coupling reactions are run in organic conditions; \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; If the temperature is raised above 837 ºc,. Conditions For Coupling Reaction.
From www.researchgate.net
Coupling reactions involving propargylic derivatives a Traditional Conditions For Coupling Reaction One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. One simple example of the coupling of reaction is the. Conditions For Coupling Reaction.
From chemistry-reaction.com
Suzuki crosscoupling Reaction Examples Mechanism Application Conditions For Coupling Reaction Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. Typically, cross coupling reactions are run in organic conditions; \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; Mechanism of the suzuki coupling. One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example. Conditions For Coupling Reaction.
From pharmacyscope.com
Diazo Coupling Reaction Pharmacy Scope Conditions For Coupling Reaction Mechanism of the suzuki coupling. One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. Typically, cross coupling reactions are run in organic conditions; Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. A coupling reaction in. Conditions For Coupling Reaction.
From encyclopedia.pub
Principles of the Suzuki Coupling Reaction Encyclopedia MDPI Conditions For Coupling Reaction Typically, cross coupling reactions are run in organic conditions; One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. One simple example of the coupling of reaction is the decomposition of calcium carbonate: If the temperature is raised above 837 ºc, this reaction becomes. A coupling. Conditions For Coupling Reaction.
From www.slideshare.net
Mechanistic aspects of CC cross coupling reaction Conditions For Coupling Reaction One simple example of the coupling of reaction is the decomposition of calcium carbonate: Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. One difference. Conditions For Coupling Reaction.
From www.researchgate.net
Reaction condition optimization for the Sonogashira coupling reaction Conditions For Coupling Reaction If the temperature is raised above 837 ºc, this reaction becomes. Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal. Conditions For Coupling Reaction.
From www.researchgate.net
Screening of reaction conditions for CO coupling a Download Table Conditions For Coupling Reaction If the temperature is raised above 837 ºc, this reaction becomes. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid. Conditions For Coupling Reaction.
From chemistnotes.com
Suzuki reaction easy mechanism,application Chemistry Notes Conditions For Coupling Reaction Typically, cross coupling reactions are run in organic conditions; One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. If the temperature is raised above 837 ºc, this reaction becomes. One simple example of the coupling of reaction is the decomposition of calcium carbonate: Transition metal. Conditions For Coupling Reaction.
From www.researchgate.net
Optimization of reaction conditions for the SuzukiMiyaura coupling Conditions For Coupling Reaction One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. If the temperature is raised above 837 ºc, this reaction becomes. \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; Typically,. Conditions For Coupling Reaction.
From nrochemistry.com
Suzuki Coupling Conditions For Coupling Reaction \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. If the temperature is raised above 837 ºc, this reaction. Conditions For Coupling Reaction.
From www.researchgate.net
Optimization of the reaction conditions for Suzuki coupling reactions Conditions For Coupling Reaction Mechanism of the suzuki coupling. If the temperature is raised above 837 ºc, this reaction becomes. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must. Conditions For Coupling Reaction.
From www.slideserve.com
PPT Buchwald _ Hart wig Cross Coupling Reaction Wala Ghannam Conditions For Coupling Reaction Mechanism of the suzuki coupling. One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. If the temperature is raised above 837. Conditions For Coupling Reaction.
From www.researchgate.net
Crosscoupling reactions employing sulfoxides as electrophiles. a Conditions For Coupling Reaction One simple example of the coupling of reaction is the decomposition of calcium carbonate: Mechanism of the suzuki coupling. One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the. Conditions For Coupling Reaction.
From thechemistrynotes.com
Coupled ReactionThermodynamics Explanation, Example Conditions For Coupling Reaction Typically, cross coupling reactions are run in organic conditions; If the temperature is raised above 837 ºc, this reaction becomes. Mechanism of the suzuki coupling. One simple example of the coupling of reaction is the decomposition of calcium carbonate: A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the. Conditions For Coupling Reaction.
From www.chemistrylearner.com
Suzuki Reaction Definition, Example, Mechanism & Application Conditions For Coupling Reaction \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; Typically, cross coupling reactions are run in organic conditions; One simple example of the coupling of reaction is the decomposition of calcium carbonate: If the temperature is raised above 837 ºc, this reaction becomes. Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. A coupling. Conditions For Coupling Reaction.
From www.youtube.com
Palladium CrossCoupling Reactions 1. An Introduction YouTube Conditions For Coupling Reaction \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. Typically, cross coupling reactions are run in organic conditions; A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the. Conditions For Coupling Reaction.
From www.researchgate.net
CuI/proline catalyzed coupling reaction of aryl bromides with secondary Conditions For Coupling Reaction A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. One simple example of the coupling of reaction is the decomposition of calcium carbonate: \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; Transition metal catalysts are used because they increase the reaction rate without affecting the. Conditions For Coupling Reaction.
From www.researchgate.net
Reaction conditions for cross coupling a Download Scientific Diagram Conditions For Coupling Reaction One simple example of the coupling of reaction is the decomposition of calcium carbonate: If the temperature is raised above 837 ºc, this reaction becomes. \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; Typically, cross coupling reactions are run in organic conditions; Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. One difference. Conditions For Coupling Reaction.
From www.researchgate.net
Substrate scope for intermolecular C−O and C−S coupling reactions Conditions For Coupling Reaction Mechanism of the suzuki coupling. Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the reaction. Typically, cross coupling reactions are run in organic conditions; One simple example of the coupling of reaction is the decomposition of calcium carbonate: A coupling reaction in organic chemistry is a general term for various reactions where. Conditions For Coupling Reaction.
From www.researchgate.net
Optimized reaction conditions for annulative coupling Conditions For Coupling Reaction Mechanism of the suzuki coupling. One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. One simple example of the. Conditions For Coupling Reaction.
From www.researchgate.net
Reaction conditions used for coupling reactions. Download Table Conditions For Coupling Reaction One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; Transition metal catalysts are. Conditions For Coupling Reaction.
From nrochemistry.com
Heck Coupling Conditions For Coupling Reaction A coupling reaction in organic chemistry is a general term for various reactions where two fragments are joined together with the aid of a metal catalyst. \[caco_{3(s)} \rightleftharpoons cao_{(s)} + co_{2(g)} \;\;\;\;\;\;\; One simple example of the coupling of reaction is the decomposition of calcium carbonate: If the temperature is raised above 837 ºc, this reaction becomes. Typically, cross coupling. Conditions For Coupling Reaction.
From www.semanticscholar.org
1,1'Carbonyldiimidazole (CDI) Mediated Coupling and Cyclization To Conditions For Coupling Reaction One difference between the suzuki mechanism and that of the stille coupling is that the boronic acid must be activated, for example with base. Mechanism of the suzuki coupling. One simple example of the coupling of reaction is the decomposition of calcium carbonate: Transition metal catalysts are used because they increase the reaction rate without affecting the heat of the. Conditions For Coupling Reaction.