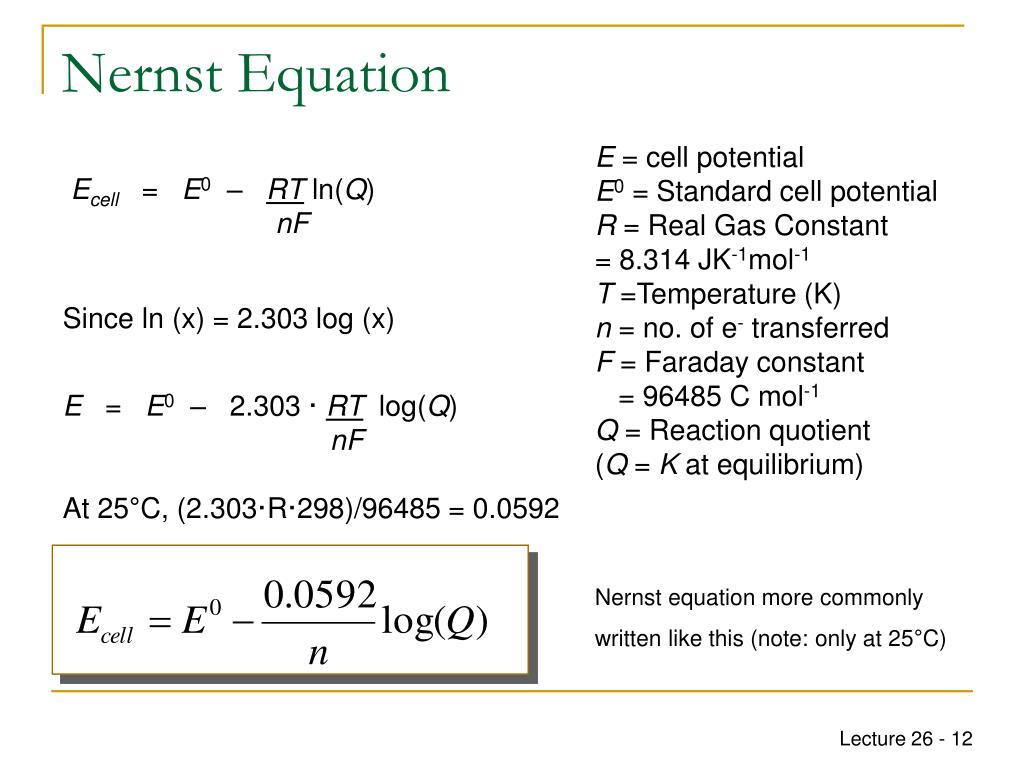
What Is R In The Nernst Equation . E° is the standard cell potential under. R = universal gas constant. Perform calculations that involve converting between. N = number of electrons transferred in the redox reaction. The nernst equation is mathematically expressed as: Use the nernst equation to determine cell potentials at nonstandard conditions; R is the gas constant used in the ideal gas law and nernst equation. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. It relates the measured cell potential to the.
from mavink.com
The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. It relates the measured cell potential to the. R = universal gas constant. Perform calculations that involve converting between. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. N = number of electrons transferred in the redox reaction. R is the gas constant used in the ideal gas law and nernst equation. E° is the standard cell potential under. Use the nernst equation to determine cell potentials at nonstandard conditions;
Nernst Equation Equilibrium Potential
What Is R In The Nernst Equation R = universal gas constant. R = universal gas constant. E° is the standard cell potential under. It relates the measured cell potential to the. The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. R is the gas constant used in the ideal gas law and nernst equation. The nernst equation is mathematically expressed as: Use the nernst equation to determine cell potentials at nonstandard conditions; Perform calculations that involve converting between. The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. N = number of electrons transferred in the redox reaction.
From www.slideserve.com
PPT From Voltage Cells to Nernst Equation PowerPoint Presentation What Is R In The Nernst Equation Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. Perform calculations that involve converting between. It relates the measured cell potential to the. R is the gas constant used in the ideal gas law and nernst equation. The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential. What Is R In The Nernst Equation.
From www.slideserve.com
PPT Using NERNST Eqn PowerPoint Presentation, free download ID3672185 What Is R In The Nernst Equation Use the nernst equation to determine cell potentials at nonstandard conditions; R = universal gas constant. N = number of electrons transferred in the redox reaction. It relates the measured cell potential to the. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. Perform calculations that involve converting between. The nernst equation is. What Is R In The Nernst Equation.
From www.youtube.com
The Nernst Equation and Concentration Cells Plus Examples YouTube What Is R In The Nernst Equation It relates the measured cell potential to the. R is the gas constant used in the ideal gas law and nernst equation. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. E° is the standard cell potential under. Use the nernst equation to determine cell potentials at nonstandard conditions; The nernst equation is. What Is R In The Nernst Equation.
From www.youtube.com
Ch 13 12 Nernst Equation for a Half and WholeCell YouTube What Is R In The Nernst Equation N = number of electrons transferred in the redox reaction. E° is the standard cell potential under. Use the nernst equation to determine cell potentials at nonstandard conditions; The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. R = universal gas constant.. What Is R In The Nernst Equation.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation, free download ID6200903 What Is R In The Nernst Equation The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. It relates the measured cell potential to the. The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. N = number. What Is R In The Nernst Equation.
From itsreleased.com
Nernst Equation Understanding Electrochemical Equilibrium What Is R In The Nernst Equation The nernst equation is mathematically expressed as: R = universal gas constant. The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. R is the gas constant used in the ideal gas law and nernst equation. Perform calculations that involve converting between. It relates the measured cell potential. What Is R In The Nernst Equation.
From general.chemistrysteps.com
Nernst Equation Chemistry Steps What Is R In The Nernst Equation R is the gas constant used in the ideal gas law and nernst equation. Perform calculations that involve converting between. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. N = number of electrons transferred in the redox reaction. The calculation of single electrode reduction potential (e red) from the standard single electrode. What Is R In The Nernst Equation.
From www.studypool.com
SOLUTION Nernst equation 2 Studypool What Is R In The Nernst Equation E° is the standard cell potential under. Use the nernst equation to determine cell potentials at nonstandard conditions; R = universal gas constant. The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. It relates the measured cell potential to the. R is the gas constant used in. What Is R In The Nernst Equation.
From www.slideserve.com
PPT Chapter 5 Biopotential Electrodes by Michael R. Neuman PowerPoint What Is R In The Nernst Equation Perform calculations that involve converting between. The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. R = universal gas constant. E° is the standard cell potential under. It relates the measured cell potential to the. R is the gas constant used in the ideal gas law and. What Is R In The Nernst Equation.
From www.slideserve.com
PPT Electrochemistry Part IV Spontaneity & Nernst Equation What Is R In The Nernst Equation N = number of electrons transferred in the redox reaction. R = universal gas constant. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. The nernst equation is mathematically expressed as: The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured.. What Is R In The Nernst Equation.
From www.youtube.com
CLASS 12 JEE & NEET ELECTROCHEMISTRY NERNST EQUATION & ELECTRODE What Is R In The Nernst Equation It relates the measured cell potential to the. The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. R is the gas constant used in the ideal gas law and nernst equation. The nernst equation allows the calculation of relative activities of the. What Is R In The Nernst Equation.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation, free download ID6621500 What Is R In The Nernst Equation Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. R is the gas constant used in the ideal gas law and nernst equation. The nernst equation is mathematically expressed as: It relates the measured cell potential to the. N = number of electrons transferred in the redox reaction. The calculation of single electrode. What Is R In The Nernst Equation.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation ID295837 What Is R In The Nernst Equation The nernst equation is mathematically expressed as: Use the nernst equation to determine cell potentials at nonstandard conditions; E° is the standard cell potential under. The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. Learn its value in different units, its symbol. What Is R In The Nernst Equation.
From www.youtube.com
AP Ch 21 Using The Nernst Equation YouTube What Is R In The Nernst Equation Perform calculations that involve converting between. R is the gas constant used in the ideal gas law and nernst equation. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. The nernst equation is mathematically expressed as: E° is the standard cell potential under. It relates the measured cell potential to the. R =. What Is R In The Nernst Equation.
From www.toppr.com
Write Nernst equation for single electrode potential at 298 K What Is R In The Nernst Equation The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. The nernst equation is mathematically expressed as: It relates the measured. What Is R In The Nernst Equation.
From www.palmsens.com
Nernst Equation explained (practical example included) What Is R In The Nernst Equation E° is the standard cell potential under. The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. R is the gas constant used in the ideal gas law and nernst equation. N = number of electrons transferred in the redox reaction. R = universal gas constant. Perform calculations. What Is R In The Nernst Equation.
From sop4cv.com
The Nernst Equation What Is R In The Nernst Equation R is the gas constant used in the ideal gas law and nernst equation. Perform calculations that involve converting between. It relates the measured cell potential to the. R = universal gas constant. E° is the standard cell potential under. The nernst equation is mathematically expressed as: Learn its value in different units, its symbol origin, and its relation to. What Is R In The Nernst Equation.
From www.youtube.com
Nernst Equation Ecell Electrochemistry Physical Chemistry What Is R In The Nernst Equation R is the gas constant used in the ideal gas law and nernst equation. The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. N = number. What Is R In The Nernst Equation.
From sop4cv.com
The Nernst Equation What Is R In The Nernst Equation Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. R is the gas constant used in the ideal gas law and nernst equation. Use the nernst equation to determine cell potentials at nonstandard conditions; The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function. What Is R In The Nernst Equation.
From www.youtube.com
Nernst equation part 1 YouTube What Is R In The Nernst Equation Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. Perform calculations that involve converting between. The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. The nernst equation allows the calculation of relative activities of the. What Is R In The Nernst Equation.
From www.slideserve.com
PPT Nernst equation PowerPoint Presentation ID6775484 What Is R In The Nernst Equation R = universal gas constant. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. E° is the standard cell potential under. The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. N = number of electrons. What Is R In The Nernst Equation.
From www.studypool.com
SOLUTION Nernst equation and calculation Studypool What Is R In The Nernst Equation Perform calculations that involve converting between. The nernst equation is mathematically expressed as: R is the gas constant used in the ideal gas law and nernst equation. N = number of electrons transferred in the redox reaction. E° is the standard cell potential under. The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential. What Is R In The Nernst Equation.
From chemistnotes.com
Nernst Equation Definition, derivation, applications Chemistry Notes What Is R In The Nernst Equation N = number of electrons transferred in the redox reaction. R is the gas constant used in the ideal gas law and nernst equation. It relates the measured cell potential to the. Use the nernst equation to determine cell potentials at nonstandard conditions; The nernst equation allows the calculation of relative activities of the species in a redox reaction as. What Is R In The Nernst Equation.
From www.youtube.com
How to use the Nernst equation graphically in the form of y = mx + c What Is R In The Nernst Equation E° is the standard cell potential under. The nernst equation is mathematically expressed as: N = number of electrons transferred in the redox reaction. It relates the measured cell potential to the. The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. Learn its value in different units,. What Is R In The Nernst Equation.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation, free download ID6600057 What Is R In The Nernst Equation The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. R is the gas constant used in the ideal gas law and nernst equation. N = number. What Is R In The Nernst Equation.
From www.youtube.com
Chem102 The Nernst Equation YouTube What Is R In The Nernst Equation Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. N = number of electrons transferred in the redox reaction. The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. R = universal gas constant. The nernst. What Is R In The Nernst Equation.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation, free download ID6600057 What Is R In The Nernst Equation R is the gas constant used in the ideal gas law and nernst equation. Perform calculations that involve converting between. The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. Use the nernst equation to determine cell potentials at nonstandard conditions; The nernst. What Is R In The Nernst Equation.
From mavink.com
Nernst Equation Equilibrium Potential What Is R In The Nernst Equation The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. Perform calculations that involve converting between. Use the nernst equation to determine cell potentials at nonstandard conditions; R is the gas constant. What Is R In The Nernst Equation.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation ID370431 What Is R In The Nernst Equation E° is the standard cell potential under. Use the nernst equation to determine cell potentials at nonstandard conditions; The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. N = number of. What Is R In The Nernst Equation.
From www.youtube.com
Using the Nernst equation Redox reactions and electrochemistry What Is R In The Nernst Equation R is the gas constant used in the ideal gas law and nernst equation. The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. Use the nernst equation to determine cell potentials at nonstandard conditions; Learn its value in different units, its symbol. What Is R In The Nernst Equation.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation, free download ID6200903 What Is R In The Nernst Equation The calculation of single electrode reduction potential (e red) from the standard single electrode reduction potential (e° red) for an atom/ion is given by the nernst equation. The nernst equation is mathematically expressed as: E° is the standard cell potential under. Use the nernst equation to determine cell potentials at nonstandard conditions; R is the gas constant used in the. What Is R In The Nernst Equation.
From scienceinfo.com
The Nernst Equation Derivation, Application, and Limitations What Is R In The Nernst Equation Use the nernst equation to determine cell potentials at nonstandard conditions; The nernst equation is mathematically expressed as: The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. E° is the standard cell potential under. It relates the measured cell potential to the. R is the gas constant. What Is R In The Nernst Equation.
From www.youtube.com
NERNST EQUATION easy way YouTube What Is R In The Nernst Equation N = number of electrons transferred in the redox reaction. Perform calculations that involve converting between. E° is the standard cell potential under. The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. Learn its value in different units, its symbol origin, and its relation to the boltzmann. What Is R In The Nernst Equation.
From swharden.com
NernstCalc Nernst Equation Calculator for Electrophysiology What Is R In The Nernst Equation E° is the standard cell potential under. The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of the measured. Use the nernst equation to determine cell potentials at nonstandard conditions; R is the gas constant used in the ideal gas law and nernst equation. The nernst equation is mathematically expressed. What Is R In The Nernst Equation.
From greenenergymaterial.com
Nernst Equation Electrochemistry Electrolysis What Is R In The Nernst Equation R = universal gas constant. Learn its value in different units, its symbol origin, and its relation to the boltzmann constant. It relates the measured cell potential to the. Use the nernst equation to determine cell potentials at nonstandard conditions; The nernst equation allows the calculation of relative activities of the species in a redox reaction as a function of. What Is R In The Nernst Equation.