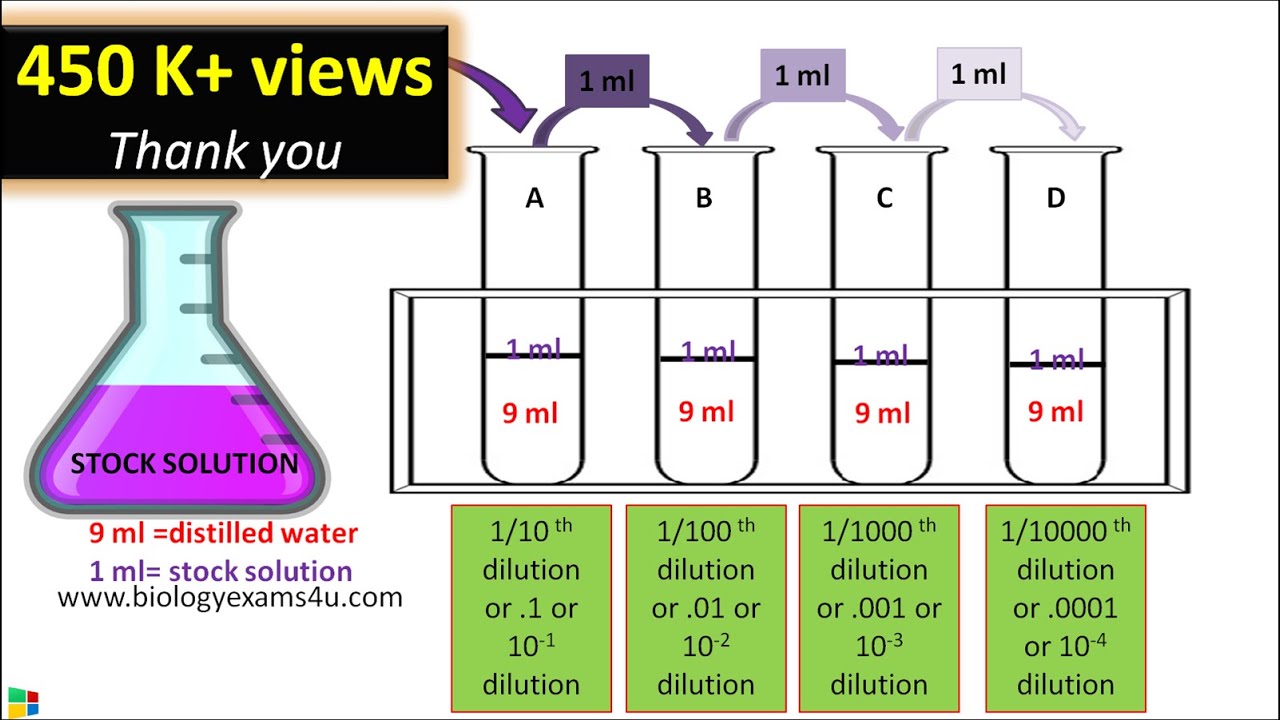
How To Do Double Dilution . Learn how to dilute and concentrate solutions. Serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent, which can either be distilled water or 0.9 %. Molarity of solution a = x molarity of solution b = y molarity of solution c = z. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute solutions. This chemistry video tutorial explains how to solve common dilution problems using a simple. Follow these five steps to make a dilution: Dilution is the addition of. If you do not know them already, use the dilution factor equation to. For example, if we mix 1.0 liter of 1.0 m glucose with 1.0 liter of distilled water, we double the. 1) assign unknowns to the final molarities of each dilution: We can do this by mixing equal volumes of our 1.00 m glucose solution with distilled water. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute.
from www.youtube.com
We can do this by mixing equal volumes of our 1.00 m glucose solution with distilled water. Follow these five steps to make a dilution: Dilution is the addition of. Learn how to dilute solutions. For example, if we mix 1.0 liter of 1.0 m glucose with 1.0 liter of distilled water, we double the. Learn how to dilute and concentrate solutions. This chemistry video tutorial explains how to solve common dilution problems using a simple. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Molarity of solution a = x molarity of solution b = y molarity of solution c = z.
Serial Dilution Method Protocol Step Wise Explanation YouTube
How To Do Double Dilution Serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent, which can either be distilled water or 0.9 %. Follow these five steps to make a dilution: We can do this by mixing equal volumes of our 1.00 m glucose solution with distilled water. 1) assign unknowns to the final molarities of each dilution: Learn how to dilute and concentrate solutions. If you do not know them already, use the dilution factor equation to. Dilution is the addition of. For example, if we mix 1.0 liter of 1.0 m glucose with 1.0 liter of distilled water, we double the. This chemistry video tutorial explains how to solve common dilution problems using a simple. Molarity of solution a = x molarity of solution b = y molarity of solution c = z. Serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent, which can either be distilled water or 0.9 %. Learn how to dilute solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute.
From www.pinterest.com
How to Do Serial Dilutions 9 Steps (with Pictures) Biology labs How To Do Double Dilution This chemistry video tutorial explains how to solve common dilution problems using a simple. Dilution is the addition of. Learn how to dilute solutions. For example, if we mix 1.0 liter of 1.0 m glucose with 1.0 liter of distilled water, we double the. If you do not know them already, use the dilution factor equation to. Follow these five. How To Do Double Dilution.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query How To Do Double Dilution This chemistry video tutorial explains how to solve common dilution problems using a simple. We can do this by mixing equal volumes of our 1.00 m glucose solution with distilled water. Follow these five steps to make a dilution: Molarity of solution a = x molarity of solution b = y molarity of solution c = z. Learn how to. How To Do Double Dilution.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube How To Do Double Dilution 1) assign unknowns to the final molarities of each dilution: Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Follow these five steps to make a dilution:. How To Do Double Dilution.
From www.researchgate.net
Procedures of serial dilution preparation Download Scientific Diagram How To Do Double Dilution Learn how to dilute and concentrate solutions. We can do this by mixing equal volumes of our 1.00 m glucose solution with distilled water. Molarity of solution a = x molarity of solution b = y molarity of solution c = z. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Learn. How To Do Double Dilution.
From www.youtube.com
AS Biology How to calculate serial and simple dilutions YouTube How To Do Double Dilution Molarity of solution a = x molarity of solution b = y molarity of solution c = z. We can do this by mixing equal volumes of our 1.00 m glucose solution with distilled water. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Often, a worker will need to change the. How To Do Double Dilution.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube How To Do Double Dilution We can do this by mixing equal volumes of our 1.00 m glucose solution with distilled water. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent, which can either be. How To Do Double Dilution.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii How To Do Double Dilution This chemistry video tutorial explains how to solve common dilution problems using a simple. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent, which can either be distilled water or 0.9. How To Do Double Dilution.
From www.slideserve.com
PPT Introduction to Serology PowerPoint Presentation, free download How To Do Double Dilution Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Learn how to dilute solutions. If you do not know them already, use the dilution factor equation to. Dilution is the addition of. We can. How To Do Double Dilution.
From zhtutorials.com
Serial Dilution Practical Skills Ep 3 Zoë Huggett Tutorials How To Do Double Dilution Molarity of solution a = x molarity of solution b = y molarity of solution c = z. Follow these five steps to make a dilution: Learn how to dilute and concentrate solutions. Learn how to dilute solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Solutions can be prepared. How To Do Double Dilution.
From www.youtube.com
How Do You Calculate Dilution Factor? YouTube How To Do Double Dilution Follow these five steps to make a dilution: Often, a worker will need to change the concentration of a solution by changing the amount of solvent. We can do this by mixing equal volumes of our 1.00 m glucose solution with distilled water. Molarity of solution a = x molarity of solution b = y molarity of solution c =. How To Do Double Dilution.
From www.laffertyequipment.com
Double Dilution Calculator Lafferty Equipment Manufacturing, Inc. How To Do Double Dilution We can do this by mixing equal volumes of our 1.00 m glucose solution with distilled water. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Learn how to dilute and concentrate solutions. Follow these five steps to make a dilution: This chemistry video tutorial explains how to solve common dilution problems. How To Do Double Dilution.
From www.slideserve.com
PPT Making Dilutions & Electrolytes PowerPoint Presentation ID6829039 How To Do Double Dilution Learn how to dilute and concentrate solutions. For example, if we mix 1.0 liter of 1.0 m glucose with 1.0 liter of distilled water, we double the. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute solutions. Molarity of solution a = x molarity of solution b. How To Do Double Dilution.
From www.slideserve.com
PPT Serial Dilutions PowerPoint Presentation ID149795 How To Do Double Dilution This chemistry video tutorial explains how to solve common dilution problems using a simple. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent, which can either be distilled water or 0.9. How To Do Double Dilution.
From www.youtube.com
HOW TO DO A 2FOLD SERIAL DILUTION? YouTube How To Do Double Dilution If you do not know them already, use the dilution factor equation to. This chemistry video tutorial explains how to solve common dilution problems using a simple. For example, if we mix 1.0 liter of 1.0 m glucose with 1.0 liter of distilled water, we double the. Learn how to dilute solutions. Serial dilution involves the process of taking a. How To Do Double Dilution.
From www.youtube.com
Chem121 Dilution Equation 8 5 YouTube How To Do Double Dilution Serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent, which can either be distilled water or 0.9 %. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. We can do this by. How To Do Double Dilution.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions How To Do Double Dilution Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. 1) assign unknowns to the final molarities of each dilution: Follow these five steps to make a dilution: For example, if we mix 1.0 liter of 1.0 m glucose with 1.0 liter of distilled water, we double. How To Do Double Dilution.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality How To Do Double Dilution 1) assign unknowns to the final molarities of each dilution: Follow these five steps to make a dilution: This chemistry video tutorial explains how to solve common dilution problems using a simple. Molarity of solution a = x molarity of solution b = y molarity of solution c = z. Learn how to dilute and concentrate solutions. Learn how to. How To Do Double Dilution.
From www.youtube.com
the simplest way to do serial dilutions (decimal & double). YouTube How To Do Double Dilution This chemistry video tutorial explains how to solve common dilution problems using a simple. 1) assign unknowns to the final molarities of each dilution: Learn how to dilute solutions. Dilution is the addition of. Molarity of solution a = x molarity of solution b = y molarity of solution c = z. Serial dilution involves the process of taking a. How To Do Double Dilution.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision How To Do Double Dilution Molarity of solution a = x molarity of solution b = y molarity of solution c = z. This chemistry video tutorial explains how to solve common dilution problems using a simple. For example, if we mix 1.0 liter of 1.0 m glucose with 1.0 liter of distilled water, we double the. Follow these five steps to make a dilution:. How To Do Double Dilution.
From www.youtube.com
12 dilution.why need this dilution.How to prepare dilution with How To Do Double Dilution Follow these five steps to make a dilution: Dilution is the addition of. Serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent, which can either be distilled water or 0.9 %. Learn how to dilute and concentrate solutions. Learn how to dilute solutions. For example, if we mix. How To Do Double Dilution.
From www.slideserve.com
PPT Serial Dilutions PowerPoint Presentation ID149795 How To Do Double Dilution This chemistry video tutorial explains how to solve common dilution problems using a simple. Molarity of solution a = x molarity of solution b = y molarity of solution c = z. Learn how to dilute solutions. Serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent, which can. How To Do Double Dilution.
From mmerevise.co.uk
Concentrations and Dilutions MME How To Do Double Dilution Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Learn how to dilute and concentrate solutions. This chemistry video tutorial explains how to solve common dilution problems using a simple. Molarity of solution a. How To Do Double Dilution.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA How To Do Double Dilution Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Follow these five steps to make a dilution: 1) assign unknowns to the final molarities of each dilution: Learn how to dilute solutions. If you do not know them already, use the dilution factor equation to. Dilution is the addition of. For example,. How To Do Double Dilution.
From www.wikihow.com
How to Do Serial Dilutions 9 Steps (with Pictures) wikiHow How To Do Double Dilution For example, if we mix 1.0 liter of 1.0 m glucose with 1.0 liter of distilled water, we double the. This chemistry video tutorial explains how to solve common dilution problems using a simple. 1) assign unknowns to the final molarities of each dilution: Molarity of solution a = x molarity of solution b = y molarity of solution c. How To Do Double Dilution.
From www.youtube.com
Calcul de dose Exercice 13 DOUBLE DILUTION YouTube How To Do Double Dilution Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. 1) assign unknowns to the final molarities of each dilution: We can do this by mixing equal volumes of our 1.00 m glucose solution with distilled water. Solutions can be prepared by diluting a more. How To Do Double Dilution.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube How To Do Double Dilution If you do not know them already, use the dilution factor equation to. Dilution is the addition of. Learn how to dilute solutions. 1) assign unknowns to the final molarities of each dilution: This chemistry video tutorial explains how to solve common dilution problems using a simple. Follow these five steps to make a dilution: For example, if we mix. How To Do Double Dilution.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew How To Do Double Dilution Dilution is the addition of. Learn how to dilute and concentrate solutions. 1) assign unknowns to the final molarities of each dilution: This chemistry video tutorial explains how to solve common dilution problems using a simple. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Serial dilution involves the process of taking. How To Do Double Dilution.
From mungfali.com
Diagrama De Diluciones How To Do Double Dilution Learn how to dilute and concentrate solutions. Follow these five steps to make a dilution: If you do not know them already, use the dilution factor equation to. Molarity of solution a = x molarity of solution b = y molarity of solution c = z. This chemistry video tutorial explains how to solve common dilution problems using a simple.. How To Do Double Dilution.
From bdaschools.weebly.com
bdaschools Blog How To Do Double Dilution Serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent, which can either be distilled water or 0.9 %. For example, if we mix 1.0 liter of 1.0 m glucose with 1.0 liter of distilled water, we double the. Dilution is the addition of. Solutions can be prepared by. How To Do Double Dilution.
From www.youtube.com
120 Dilution.Two easy methods to prepare.learn & understand then can How To Do Double Dilution Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Learn how to dilute and concentrate solutions. 1) assign unknowns to the final molarities of each dilution: Learn how to dilute solutions. Serial dilution involves the process of taking a sample and diluting it through a series of standard volumes of sterile diluent,. How To Do Double Dilution.
From www.showme.com
Dilution calculation Science, Chemicalreactions, Biology ShowMe How To Do Double Dilution Follow these five steps to make a dilution: Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. 1) assign unknowns to the final molarities of each dilution: Molarity of solution a = x molarity of solution b = y molarity of solution c = z. For example, if we mix 1.0 liter. How To Do Double Dilution.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube How To Do Double Dilution Learn how to dilute and concentrate solutions. Follow these five steps to make a dilution: Learn how to dilute solutions. For example, if we mix 1.0 liter of 1.0 m glucose with 1.0 liter of distilled water, we double the. Solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. We can do. How To Do Double Dilution.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved How To Do Double Dilution If you do not know them already, use the dilution factor equation to. 1) assign unknowns to the final molarities of each dilution: Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. Serial dilution involves the process of taking a sample and diluting it through a. How To Do Double Dilution.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube How To Do Double Dilution Follow these five steps to make a dilution: For example, if we mix 1.0 liter of 1.0 m glucose with 1.0 liter of distilled water, we double the. We can do this by mixing equal volumes of our 1.00 m glucose solution with distilled water. Solutions can be prepared by diluting a more concentrated solution rather than starting with the. How To Do Double Dilution.
From chem370.github.io
Lab 1 Prelab CHEM 370 How To Do Double Dilution Follow these five steps to make a dilution: Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Molarity of solution a = x molarity of solution b = y molarity of solution c = z. Learn how to dilute solutions. For example, if we mix 1.0 liter of 1.0 m glucose. How To Do Double Dilution.