What Is Another Name For Law Of Definite Proportions . The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of a pure compound always contain the same elements in. The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. The law of definite proportions is. The law of definite proportions states that a chemically pure substance always contains the same set of elements combined. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass.
from studylib.net
Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions states that a chemically pure substance always contains the same set of elements combined. The law of definite proportions is. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of a pure compound always contain the same elements in. The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry.
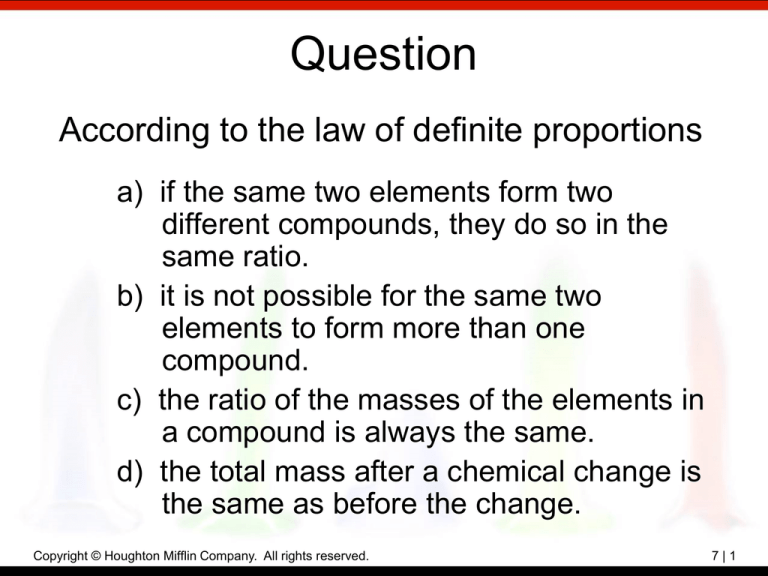
Question According to the law of definite proportions
What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a chemically pure substance always contains the same set of elements combined. The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. The law of definite proportions is. Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. The law of definite proportions states that a chemically pure substance always contains the same set of elements combined. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of a pure compound always contain the same elements in.
From www.youtube.com
Law Of Definite Proportion law of chemical combination Chemistry What Is Another Name For Law Of Definite Proportions The law of definite proportions is. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of a pure compound always contain the same elements in. The law of. What Is Another Name For Law Of Definite Proportions.
From www.youtube.com
Law of definite Proportion Vs Law of Multiple Proportion class 9th,11th What Is Another Name For Law Of Definite Proportions Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. The law of definite proportions states that a chemically pure substance always contains the same set of elements combined. The law of. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT Definite Proportions, Multiple Proportions and Atomic Theory What Is Another Name For Law Of Definite Proportions Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of a pure compound always contain the same elements in. The law of definite proportions states that a given chemical compound always contains. What Is Another Name For Law Of Definite Proportions.
From www.youtube.com
Law of Definite Proportions Chemistry Practice Problems Chemical What Is Another Name For Law Of Definite Proportions The law of definite proportions is. The law of definite proportions states that a chemically pure substance always contains the same set of elements combined. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of a pure compound always contain the same elements in. The law of definite proportions states that. What Is Another Name For Law Of Definite Proportions.
From www.youtube.com
Law of Definite Proportion Chemistry Basics YouTube What Is Another Name For Law Of Definite Proportions Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions states that a chemically pure substance always contains the same set of elements combined. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID408295 What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT Law of Definite Proportions PowerPoint Presentation, free What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by. What Is Another Name For Law Of Definite Proportions.
From www.expii.com
Proust's Law of Definite Proportions — Overview & Origin Expii What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a chemically pure substance always contains the same set of elements combined. The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT Empirical and Molecular Formulas PowerPoint Presentation, free What Is Another Name For Law Of Definite Proportions The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. The law of definite proportions is. The law of definite proportions states that a chemically pure substance always contains. What Is Another Name For Law Of Definite Proportions.
From gamesmartz.com
Law Of Definite Proportion Definition & Image GameSmartz What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT Laws of Definite Proportions & Multiple Proportions PowerPoint What Is Another Name For Law Of Definite Proportions Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions is. The law of definite proportions states that a chemically pure substance always contains the same set of elements combined. The law of definite proportions states that a given chemical compound always contains the same elements. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT The Law of Definite Proportions PowerPoint Presentation, free What Is Another Name For Law Of Definite Proportions In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of a pure compound always contain the same elements in. The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. The law of definite proportions states that a chemically pure. What Is Another Name For Law Of Definite Proportions.
From www.youtube.com
What is the Law of Definite Proportion? YouTube What Is Another Name For Law Of Definite Proportions Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. The law of definite proportions is. The law of definite proportions, together with the law of multiple proportions,. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT Chapter 1 PowerPoint Presentation, free download ID6426283 What Is Another Name For Law Of Definite Proportions Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT Laws of Definite Proportions & Multiple Proportions PowerPoint What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a chemically pure substance always contains the same set of elements combined. The law of definite proportions is. Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions, together with the law of multiple proportions, forms the basis for. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT Lavoisier The Law of Conservation of Mass PowerPoint What Is Another Name For Law Of Definite Proportions The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of. What Is Another Name For Law Of Definite Proportions.
From ar.inspiredpencil.com
Law Of Definite Proportions What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a chemically pure substance always contains the same set of elements combined. The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT Definite proportions and percent by mass PowerPoint Presentation What Is Another Name For Law Of Definite Proportions The law of definite proportions is. The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. Law of definite proportions, statement that every chemical compound contains fixed and constant. What Is Another Name For Law Of Definite Proportions.
From studylib.net
Question According to the law of definite proportions What Is Another Name For Law Of Definite Proportions Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT The Law of Definite Proportions PowerPoint Presentation, free What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a chemically pure substance always contains the same set of elements combined. The law of definite proportions is. The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. The law of definite proportions states that a compound always contains exactly the. What Is Another Name For Law Of Definite Proportions.
From sciencenotes.org
Law of Definite Proportions Law of Constant Composition What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions states that a chemically pure substance always contains the same set of elements. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT Definite Proportions, Multiple Proportions and Atomic Theory What Is Another Name For Law Of Definite Proportions In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of a pure compound always contain the same elements in. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. The law of definite proportions states that a chemically. What Is Another Name For Law Of Definite Proportions.
From slidetodoc.com
Definite Proportions Multiple Proportions and Atomic Theory Law What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. The law of definite proportions states that a chemically pure substance always contains the same set of elements. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT The Law of Definite Proportions PowerPoint Presentation, free What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a chemically pure substance always contains the same set of elements combined. The law of definite proportions is. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of a pure compound always contain the same elements in. The law of definite proportions states that. What Is Another Name For Law Of Definite Proportions.
From kannanchemistry.wordpress.com
Law of Definite Proportions Kannan Chemistry An Online Free What Is Another Name For Law Of Definite Proportions Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions is. The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. The law of definite proportions states that a given chemical compound always contains. What Is Another Name For Law Of Definite Proportions.
From www.slideshare.net
Chapter 3 chemistry What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. The law of definite proportions states that a compound always contains exactly the same proportion. What Is Another Name For Law Of Definite Proportions.
From drcalef.com
9.1 Law of Definite Proportions What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of a pure compound always contain the same elements in. Law of definite proportions, statement that every chemical compound. What Is Another Name For Law Of Definite Proportions.
From www.teachmint.com
Law Of Definite Proportion Chemistry Notes Teachmint What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. The law of definite proportions states that a compound always contains exactly the same proportion. What Is Another Name For Law Of Definite Proportions.
From printablelibwaneer.z19.web.core.windows.net
Law Of Definite Proportions Worksheet What Is Another Name For Law Of Definite Proportions The law of definite proportions is. The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of a pure compound always contain the same elements in. Law of definite proportions, statement that every. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT The Law of Definite Proportions PowerPoint Presentation, free What Is Another Name For Law Of Definite Proportions The law of definite proportions is. Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. The law of definite proportions states that a compound always contains exactly the. What Is Another Name For Law Of Definite Proportions.
From byjus.com
what is law of definite proportions? What Is Another Name For Law Of Definite Proportions The law of definite proportions is. The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. The law of definite proportions states that a compound. What Is Another Name For Law Of Definite Proportions.
From www.showme.com
Law of definite and multiple proportions Science, Chemistry What Is Another Name For Law Of Definite Proportions The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry. Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions states that a chemically pure substance always contains the same set of elements combined.. What Is Another Name For Law Of Definite Proportions.
From slideplayer.com
Laws of Definite Proportions & Multiple Proportions ppt download What Is Another Name For Law Of Definite Proportions The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. The law of definite proportions, together with the law of multiple proportions, forms the basis for the study of stoichiometry in chemistry.. What Is Another Name For Law Of Definite Proportions.
From www.sliderbase.com
Atoms and their structure Presentation Chemistry What Is Another Name For Law Of Definite Proportions The law of definite proportions is. The law of definite proportions states that a compound always contains exactly the same proportion of elements by mass. The law of definite proportions states that a given chemical compound always contains the same elements in the exact same proportions by mass. The law of definite proportions states that a chemically pure substance always. What Is Another Name For Law Of Definite Proportions.
From www.slideserve.com
PPT Laws of Definite Proportions & Multiple Proportions PowerPoint What Is Another Name For Law Of Definite Proportions Law of definite proportions, statement that every chemical compound contains fixed and constant proportions (by mass) of its constituent. In chemistry, the law of constant composition (also known as the law of definite proportions) states that samples of a pure compound always contain the same elements in. The law of definite proportions, together with the law of multiple proportions, forms. What Is Another Name For Law Of Definite Proportions.