Physical Test Method Validation . Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. What is test method validation for medical devices? Certain performance characteristics, such as linearity, will not vary. International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. What is the basic idea of test method validation (tmv)? The validation study must contain all pertinent performance characteristics. This test will help you assess how much. The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. Why do we have to perform test method validation (tmv)?. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and.
from www.slideserve.com
Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. This test will help you assess how much. Why do we have to perform test method validation (tmv)?. What is the basic idea of test method validation (tmv)? Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. What is test method validation for medical devices? The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. The validation study must contain all pertinent performance characteristics. Certain performance characteristics, such as linearity, will not vary.
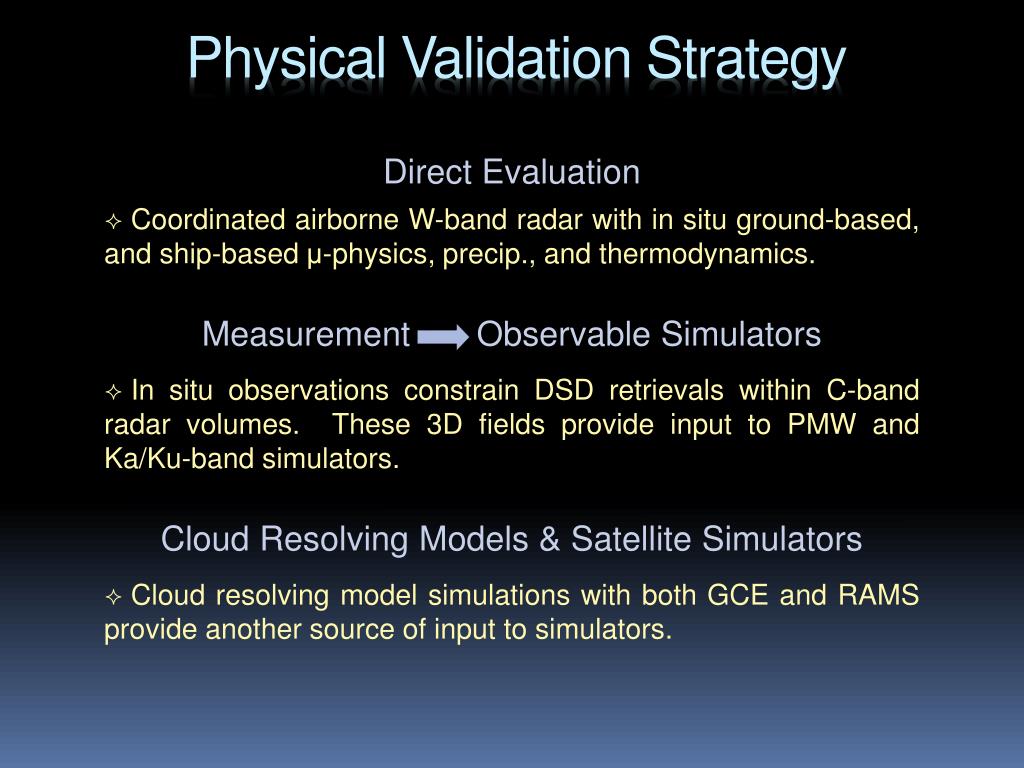
PPT Light Precipitation Validation Experiment PowerPoint Presentation
Physical Test Method Validation Certain performance characteristics, such as linearity, will not vary. Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. This test will help you assess how much. What is the basic idea of test method validation (tmv)? Certain performance characteristics, such as linearity, will not vary. What is test method validation for medical devices? The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. Why do we have to perform test method validation (tmv)?. The validation study must contain all pertinent performance characteristics.
From testingbuddy.co.in
Vmodel/ V and V model /Verification and Validation model Testingbuddy Physical Test Method Validation Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. The validation study must contain all pertinent performance characteristics. Why do we have to perform test method validation (tmv)?. This test will. Physical Test Method Validation.
From www.youtube.com
Physical Ability Testing PostOffer Employment Test CNS Physical Test Method Validation The validation study must contain all pertinent performance characteristics. Why do we have to perform test method validation (tmv)?. What is the basic idea of test method validation (tmv)? International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. What is test method validation for medical devices? Methods requiring validation may include those used for. Physical Test Method Validation.
From www.eetindia.co.in
IC validator certified for signoff physical verification EE Times India Physical Test Method Validation The validation study must contain all pertinent performance characteristics. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. This test will help you assess how much. Why do we have to perform test method validation (tmv)?. Methods requiring validation may include those used for testing of drug substance/api (potency,. Physical Test Method Validation.
From www.researchgate.net
The overall workflow for validation of the reconstructed... Download Physical Test Method Validation What is the basic idea of test method validation (tmv)? Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. The validation study must contain all pertinent performance characteristics. Why do we have to perform test method validation (tmv)?. What is test method validation for medical devices? Certain performance characteristics, such. Physical Test Method Validation.
From www.researchgate.net
(PDF) Principles of developing physical test methods for disposable Physical Test Method Validation Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. This test will help you assess how much. Certain performance characteristics, such as linearity, will not vary. The validation study must. Physical Test Method Validation.
From www.researchgate.net
Validation process of the Outpatient Physical Therapy Improvement in Physical Test Method Validation What is test method validation for medical devices? The validation study must contain all pertinent performance characteristics. What is the basic idea of test method validation (tmv)? The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that. Physical Test Method Validation.
From deborahhindi.com
Medical Device Validation Protocol Example Physical Test Method Validation The validation study must contain all pertinent performance characteristics. International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. Certain performance characteristics, such as linearity, will not vary. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. Why do we have to perform test. Physical Test Method Validation.
From here2grow.com
Analytical Testing Here2Grow Cosmetics & Homecare Labs U.K. Physical Test Method Validation Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. The validation study must contain all pertinent performance characteristics. What is the basic idea of test method validation (tmv)? The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. This test. Physical Test Method Validation.
From www.slideserve.com
PPT Instrumental Analysis PowerPoint Presentation, free download ID Physical Test Method Validation International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. The validation study must contain all pertinent performance characteristics. Test method validation is the practice of gathering objective evidence that the test fulfills its part in. Physical Test Method Validation.
From present5.com
Validation of Analytical Methods Hua YIN Outline Physical Test Method Validation Why do we have to perform test method validation (tmv)?. What is the basic idea of test method validation (tmv)? Certain performance characteristics, such as linearity, will not vary. The validation study must contain all pertinent performance characteristics. What is test method validation for medical devices? The most common approach for validating a test method is running a gage repeatability. Physical Test Method Validation.
From www.gsetech.co.in
Home to GSE Physical Test Method Validation The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. This test will help you assess how much. International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. What is test method validation for medical devices? The validation study must contain all pertinent performance characteristics. Certain. Physical Test Method Validation.
From www.slideserve.com
PPT Physical Assessment Skills PowerPoint Presentation, free download Physical Test Method Validation What is test method validation for medical devices? What is the basic idea of test method validation (tmv)? Certain performance characteristics, such as linearity, will not vary. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. The validation study must contain all pertinent performance characteristics. The most common approach. Physical Test Method Validation.
From www.slideserve.com
PPT Light Precipitation Validation Experiment PowerPoint Presentation Physical Test Method Validation Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. This test will help you assess how much. What is test method validation for medical devices? What is the basic idea. Physical Test Method Validation.
From www.xenonstack.com
Data Validation Testing Tools and Techniques Complete Guide Physical Test Method Validation International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. What is the basic idea of test method validation (tmv)? Certain performance characteristics, such as linearity, will not vary. Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. Why do we have to perform test. Physical Test Method Validation.
From www.slideshare.net
Developing and Validating “Work Sample” Physical Ability Tests (Overv… Physical Test Method Validation This test will help you assess how much. Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. The validation study must contain all pertinent performance characteristics. What is the basic idea of test method validation (tmv)? Why do we have to perform test method validation (tmv)?. Test method validation is. Physical Test Method Validation.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Physical Test Method Validation What is the basic idea of test method validation (tmv)? Why do we have to perform test method validation (tmv)?. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. This. Physical Test Method Validation.
From www.slideserve.com
PPT Instrumental Analysis PowerPoint Presentation, free download ID Physical Test Method Validation Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. Why do we have to perform test method validation (tmv)?. Certain performance characteristics, such as linearity, will not vary. This test. Physical Test Method Validation.
From www.researchgate.net
Summary of Analytical Method Validation and Stability Data of BPB and Physical Test Method Validation What is the basic idea of test method validation (tmv)? What is test method validation for medical devices? This test will help you assess how much. Why do we have to perform test method validation (tmv)?. International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. Methods requiring validation may include those used for testing. Physical Test Method Validation.
From www.youtube.com
Skills Validation Physical Assessment YouTube Physical Test Method Validation The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. What is the basic idea of test method validation (tmv)? What is test method validation for medical devices? This test will help. Physical Test Method Validation.
From www.youtube.com
Method Validation Fitness for purpose of analytical methods Part 2 Physical Test Method Validation Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. Why do we have to perform test method validation (tmv)?. Certain performance characteristics, such as linearity, will not vary. What is the. Physical Test Method Validation.
From www.slideserve.com
PPT CSS Compliance Testing Project PowerPoint Presentation, free Physical Test Method Validation What is test method validation for medical devices? The validation study must contain all pertinent performance characteristics. International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. Certain performance characteristics, such as linearity, will not vary. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety. Physical Test Method Validation.
From www.slideshare.net
Digital Validation Physical Validation Your Physical Test Method Validation Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. This test will help you assess how much. International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. What is test method validation for medical devices? Test method validation is the practice of gathering objective evidence. Physical Test Method Validation.
From www.presentationeze.com
Process Validation vs Verification. When to Validate. When to Verify Physical Test Method Validation What is the basic idea of test method validation (tmv)? International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. This test will help you assess how much. Certain performance characteristics, such as linearity, will not. Physical Test Method Validation.
From www.comsol.de
MED Institute Performs Virtual Testing and Validation of Medical Devices Physical Test Method Validation The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. What is the basic idea of test method validation (tmv)? Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. This test will help you assess how much. International standards such. Physical Test Method Validation.
From www.researchgate.net
Simplified view of the model verification and validation process. [8 Physical Test Method Validation Why do we have to perform test method validation (tmv)?. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. This test will help you assess how much. Certain performance characteristics, such as linearity, will. Physical Test Method Validation.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Physical Test Method Validation The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. What is. Physical Test Method Validation.
From slcontrols.com
The 3 Stages of Process Validation Explained SL Controls Physical Test Method Validation What is test method validation for medical devices? This test will help you assess how much. The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. Methods requiring validation may include. Physical Test Method Validation.
From www.slideserve.com
PPT Production and Process validation PowerPoint Presentation, free Physical Test Method Validation The validation study must contain all pertinent performance characteristics. The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. What is the basic idea of test method validation (tmv)? What is test method validation for medical devices? Test method validation is the practice of gathering objective evidence that the test fulfills. Physical Test Method Validation.
From www.slideshare.net
Developing and Validating “Work Sample” Physical Ability Tests (Overv… Physical Test Method Validation International standards such as iso/iec 17025, certifying bodies, and regulatory agencies require evidence that analytical. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. Why do we have to perform test method validation (tmv)?. What is the basic idea of test method validation (tmv)? What is test method validation. Physical Test Method Validation.
From www.researchgate.net
(PDF) The physical therapy profile questionnaire (PTPQ) Development Physical Test Method Validation The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. The validation study must contain all pertinent performance characteristics. Certain performance characteristics, such as linearity, will not vary. What is test method validation for medical devices? Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity,. Physical Test Method Validation.
From www.slideshare.net
1 Reliability and Validity in Physical Therapy Tests Physical Test Method Validation Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. Certain performance characteristics, such as linearity, will not vary. Why do we have to perform test method validation (tmv)?. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. This test. Physical Test Method Validation.
From www.dober.com
Cleaning Validation Support For Critical Cleaners Physical Test Method Validation Certain performance characteristics, such as linearity, will not vary. The most common approach for validating a test method is running a gage repeatability and reproducibility (gage r&r) study. Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. What is test method validation for medical devices? International standards such as iso/iec. Physical Test Method Validation.
From www.gaston.edu
Physical Testing Textile Technology Center Physical Test Method Validation What is the basic idea of test method validation (tmv)? Methods requiring validation may include those used for testing of drug substance/api (potency, impurities, identity, and other critical attributes. Certain performance characteristics, such as linearity, will not vary. This test will help you assess how much. The validation study must contain all pertinent performance characteristics. International standards such as iso/iec. Physical Test Method Validation.
From www.kewaunee.in
The VModel Approach to Lab Validation Explained Kewaunee Physical Test Method Validation Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. Why do we have to perform test method validation (tmv)?. What is test method validation for medical devices? What is the basic idea of test method validation (tmv)? The validation study must contain all pertinent performance characteristics. International standards such. Physical Test Method Validation.
From www.sofpromed.com
An Introduction to Analytical Method Development and Validation in Physical Test Method Validation This test will help you assess how much. Test method validation is the practice of gathering objective evidence that the test fulfills its part in ensuring safety and. Why do we have to perform test method validation (tmv)?. What is test method validation for medical devices? What is the basic idea of test method validation (tmv)? The validation study must. Physical Test Method Validation.