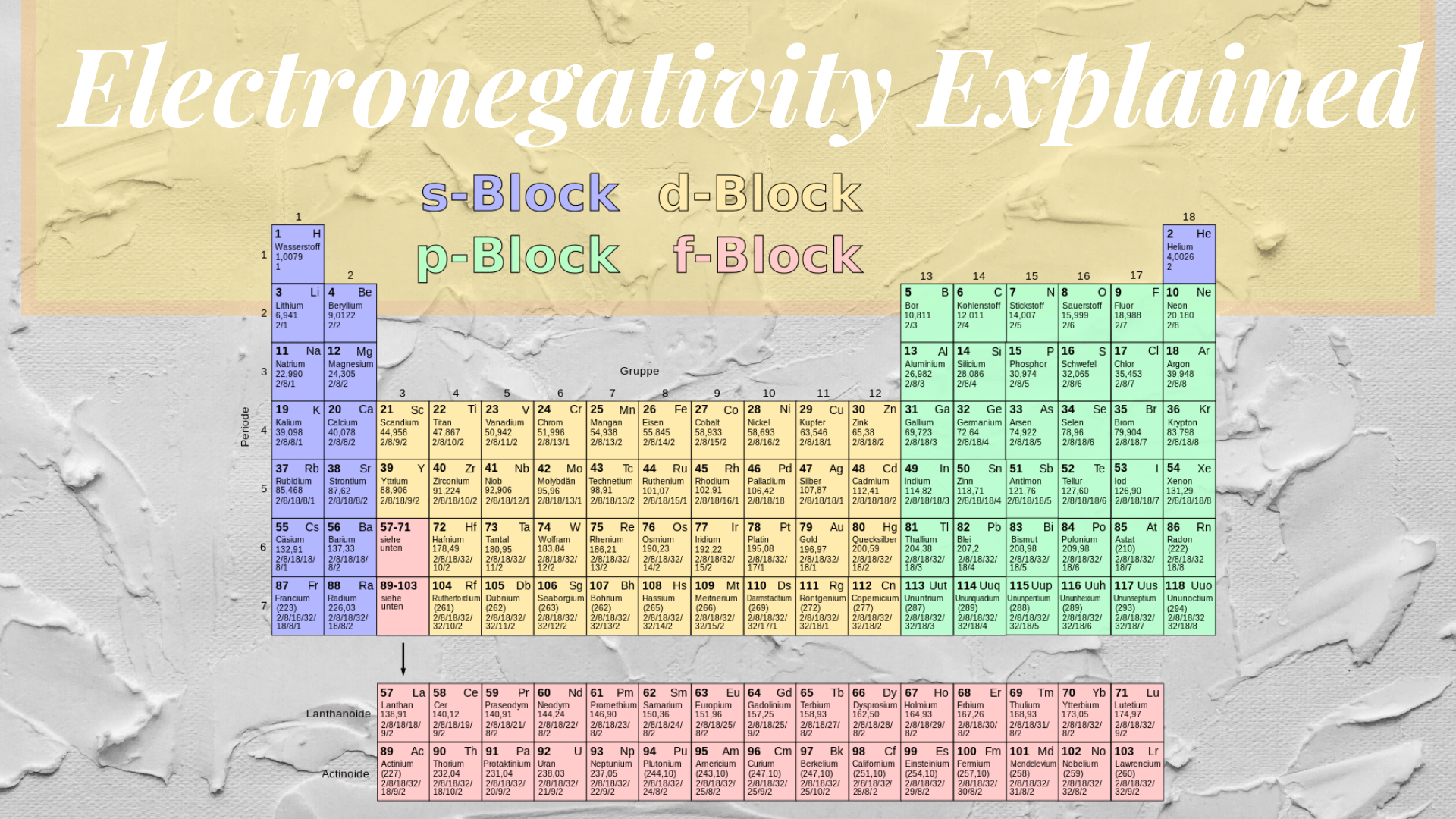
Electronegativity Of Chlorine And Carbon . Bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. This table is a list of electronegativity values of the elements. It can also be used to predict if the resulting molecule will be polar or nonpolar. When the difference is very small or zero, the bond is covalent and nonpolar. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. When it is large, the bond is polar covalent or ionic. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). It is among the highly reactive non. Since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on the periodic table, known as the halogens.
from sciencetrends.com
It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: Bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. When the difference is very small or zero, the bond is covalent and nonpolar. It is among the highly reactive non. It can also be used to predict if the resulting molecule will be polar or nonpolar. This table is a list of electronegativity values of the elements. Since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). 3.16 is the electronegativity value of chlorine (cl).
Electronegativity Chart Science Trends
Electronegativity Of Chlorine And Carbon The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: When it is large, the bond is polar covalent or ionic. When the difference is very small or zero, the bond is covalent and nonpolar. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. It can also be used to predict if the resulting molecule will be polar or nonpolar. 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. This table is a list of electronegativity values of the elements. It is among the highly reactive non. The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: Bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\).
From www.pw.live
Electronegativity Formula Introduction And Patterns In Periodic Table Electronegativity Of Chlorine And Carbon 3.16 is the electronegativity value of chlorine (cl). When it is large, the bond is polar covalent or ionic. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It is among the highly reactive non.. Electronegativity Of Chlorine And Carbon.
From sciencetrends.com
Electronegativity Chart Science Trends Electronegativity Of Chlorine And Carbon The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). It is among the highly reactive non. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or. Electronegativity Of Chlorine And Carbon.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Electronegativity Of Chlorine And Carbon It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. When it is large, the bond is polar covalent or ionic. Since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. The two chlorine atoms share the pair of electrons in. Electronegativity Of Chlorine And Carbon.
From www.animalia-life.club
Electronegativity Periodic Table 3d Electronegativity Of Chlorine And Carbon When the difference is very small or zero, the bond is covalent and nonpolar. It is among the highly reactive non. It can also be used to predict if the resulting molecule will be polar or nonpolar. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. 119 rows electronegativity is used to predict whether a bond between. Electronegativity Of Chlorine And Carbon.
From mungfali.com
Periodic Table Of Elements Electronegativity Electronegativity Of Chlorine And Carbon The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. This table is a list of electronegativity values of the elements. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Bonds between carbon and more. Electronegativity Of Chlorine And Carbon.
From cadscaleschart.z28.web.core.windows.net
electronegativity chart scale The periodic table and periodic trends Electronegativity Of Chlorine And Carbon The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). This table is a list of electronegativity values of the elements. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Chlorine's larger size decreases the effectiveness of orbital overlap in. Electronegativity Of Chlorine And Carbon.
From neurotext.library.stonybrook.edu
Cellular Neurophysiology Electronegativity Of Chlorine And Carbon The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: This table is a list of electronegativity values of the elements. Bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. It is among the highly reactive non. When the. Electronegativity Of Chlorine And Carbon.
From www.geeksforgeeks.org
Electronegativity Definition, Meaning, Periodic Trends, Examples Electronegativity Of Chlorine And Carbon When the difference is very small or zero, the bond is covalent and nonpolar. Since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. It is among the highly reactive non. 3.16 is the electronegativity value of chlorine (cl). The two chlorine atoms share the pair of electrons. Electronegativity Of Chlorine And Carbon.
From mmerevise.co.uk
Electronegativity & Intermolecular Forces MME Electronegativity Of Chlorine And Carbon When it is large, the bond is polar covalent or ionic. It can also be used to predict if the resulting molecule will be polar or nonpolar. The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It. Electronegativity Of Chlorine And Carbon.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Electronegativity Of Chlorine And Carbon Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). This table is a list of electronegativity values of the elements. It can also be used to predict if the resulting molecule will be polar or. Electronegativity Of Chlorine And Carbon.
From www.numerade.com
SOLVED Is CCla a polar or nonpolar molecule and why? Electronegativity Electronegativity Of Chlorine And Carbon When the difference is very small or zero, the bond is covalent and nonpolar. This table is a list of electronegativity values of the elements. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 3.16 is the electronegativity value of chlorine (cl). Chlorine's larger size decreases the effectiveness of orbital overlap in. Electronegativity Of Chlorine And Carbon.
From www.shutterstock.com
Electronegativity Table Elements Color Code Stock Vector (Royalty Free Electronegativity Of Chlorine And Carbon 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). When the difference is very small or zero, the bond is covalent and. Electronegativity Of Chlorine And Carbon.
From www.slideshare.net
Electronegativity part two Electronegativity Of Chlorine And Carbon It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. This table is a list of electronegativity values of the elements. When the difference is very small or zero, the bond is covalent and nonpolar. It is among the highly reactive non. Since chlorine is about as electronegative as nitrogen, the effect of. Electronegativity Of Chlorine And Carbon.
From dona.tompkinscountystructuralracism.org
Electronegativity Values Chart Understanding The Chemistry Of Elements Electronegativity Of Chlorine And Carbon It is among the highly reactive non. 3.16 is the electronegativity value of chlorine (cl). Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: This table is a list of electronegativity values of the elements. It can also be used to predict. Electronegativity Of Chlorine And Carbon.
From www.thoughtco.com
Printable Periodic Table of the Elements Electronegativity Electronegativity Of Chlorine And Carbon When the difference is very small or zero, the bond is covalent and nonpolar. The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: It can also be used to predict if the resulting molecule will be polar or nonpolar. 3.16 is the electronegativity value of chlorine (cl). The two chlorine atoms share the pair. Electronegativity Of Chlorine And Carbon.
From knordslearning.com
Electronegativity Chart of all Elements (With Periodic table) Electronegativity Of Chlorine And Carbon It can also be used to predict if the resulting molecule will be polar or nonpolar. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). Bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized. Electronegativity Of Chlorine And Carbon.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Electronegativity Of Chlorine And Carbon Bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. 3.16 is the electronegativity value of chlorine (cl). The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). When it is large, the. Electronegativity Of Chlorine And Carbon.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Electronegativity Of Chlorine And Carbon The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: 3.16 is the electronegativity value of chlorine (cl). Since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. When it is large, the bond is polar covalent or ionic. This table is a. Electronegativity Of Chlorine And Carbon.
From general.chemistrysteps.com
Electronegativity and Bond Polarity Chemistry Steps Electronegativity Of Chlorine And Carbon It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. It is among the highly reactive non. It can also be used to predict if the resulting molecule will be polar or nonpolar. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Electronegativity Of Chlorine And Carbon.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Electronegativity Of Chlorine And Carbon The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). It can also be used to predict if the resulting molecule will be polar or nonpolar. When it is large, the bond is polar covalent or ionic. The substituent δekt values are in qualitative agreement with what may. Electronegativity Of Chlorine And Carbon.
From mungfali.com
Atom Electronegativity Chart Electronegativity Of Chlorine And Carbon Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: Since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. It can also be used to predict if the resulting molecule. Electronegativity Of Chlorine And Carbon.
From mungfali.com
Electronegativity Chart And Lewis Structures Electronegativity Of Chlorine And Carbon It is among the highly reactive non. This table is a list of electronegativity values of the elements. Since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. 3.16 is the electronegativity value of chlorine (cl). Bonds between carbon and more electronegative elements such as oxygen (en =. Electronegativity Of Chlorine And Carbon.
From www.chemtopper.com
Video quiz on electronegativity part 4 on polarity in organic molecules Electronegativity Of Chlorine And Carbon It is among the highly reactive non. Since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. This table is a list of electronegativity values of the elements. When it is large, the bond is polar covalent or ionic. Chlorine's larger size decreases the effectiveness of orbital overlap. Electronegativity Of Chlorine And Carbon.
From www.numerade.com
SOLVED The electronegativity of Carbon is 2.5 and that of Chlorine is Electronegativity Of Chlorine And Carbon The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: 3.16 is the electronegativity value of chlorine (cl). 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It is among the highly reactive non. It belongs to the 7th group and 2nd period on the periodic table,. Electronegativity Of Chlorine And Carbon.
From mavink.com
Periodic Table With Electronegativity Values Electronegativity Of Chlorine And Carbon 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). When the difference is very small. Electronegativity Of Chlorine And Carbon.
From ar.inspiredpencil.com
Periodic Table With Electronegativity And Electron Configuration Electronegativity Of Chlorine And Carbon 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. This table is a list of electronegativity values of the elements. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. 3.16 is the. Electronegativity Of Chlorine And Carbon.
From www.numerade.com
SOLVED Using the electronegativity chart, identify the bond type Electronegativity Of Chlorine And Carbon The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: 3.16 is the electronegativity value of chlorine (cl). Bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. It belongs to the 7th group and 2nd period on the periodic. Electronegativity Of Chlorine And Carbon.
From cadscaleschart.z28.web.core.windows.net
electronegativity chart scale The periodic table and periodic trends Electronegativity Of Chlorine And Carbon 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. When it is large, the bond is polar covalent or ionic. Bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen. Electronegativity Of Chlorine And Carbon.
From fr.dreamstime.com
Table Périodique D'Electronegativity Image stock Image 38153931 Electronegativity Of Chlorine And Carbon Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. When it is large, the bond is polar covalent or ionic. 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. When the difference is very small or zero, the bond is covalent. Electronegativity Of Chlorine And Carbon.
From www.ck12.org
Periodic Trends in Electronegativity CK12 Foundation Electronegativity Of Chlorine And Carbon It is among the highly reactive non. The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Chlorine's larger size decreases the effectiveness of orbital overlap in bonding, and. Bonds between carbon and more electronegative elements such as. Electronegativity Of Chlorine And Carbon.
From studyafrikander.z13.web.core.windows.net
How To Determine Electronegativity Electronegativity Of Chlorine And Carbon Since chlorine is about as electronegative as nitrogen, the effect of a chlorine or a nitrogen on an attached carbon are similar. 3.16 is the electronegativity value of chlorine (cl). The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: 119 rows electronegativity is used to predict whether a bond between atoms will be ionic. Electronegativity Of Chlorine And Carbon.
From www.bigstockphoto.com
Electronegativity Vector & Photo (Free Trial) Bigstock Electronegativity Of Chlorine And Carbon It is among the highly reactive non. This table is a list of electronegativity values of the elements. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It can also be used to predict if. Electronegativity Of Chlorine And Carbon.
From www.dreamstime.com
Chlorine Chemical Element with 17 Atomic Number, Atomic Mass and Electronegativity Of Chlorine And Carbon It can also be used to predict if the resulting molecule will be polar or nonpolar. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. This table is a list of electronegativity values of the elements. It is among the highly reactive non. It belongs to the 7th group and 2nd period. Electronegativity Of Chlorine And Carbon.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF Electronegativity Of Chlorine And Carbon The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the \(\ce{cl_2}\). This table is a list of electronegativity values of the elements. 3.16 is the electronegativity value of chlorine (cl). Chlorine's larger size decreases. Electronegativity Of Chlorine And Carbon.
From chemicalbondinglife.blogspot.com
Chemical Bonding Periodic Trends Electronegativity Of Chlorine And Carbon The substituent δekt values are in qualitative agreement with what may be anticipated from electronegativity: Bonds between carbon and more electronegative elements such as oxygen (en = 3.5) and nitrogen (en = 3.0), by contrast, are polarized so that the. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding. Electronegativity Of Chlorine And Carbon.