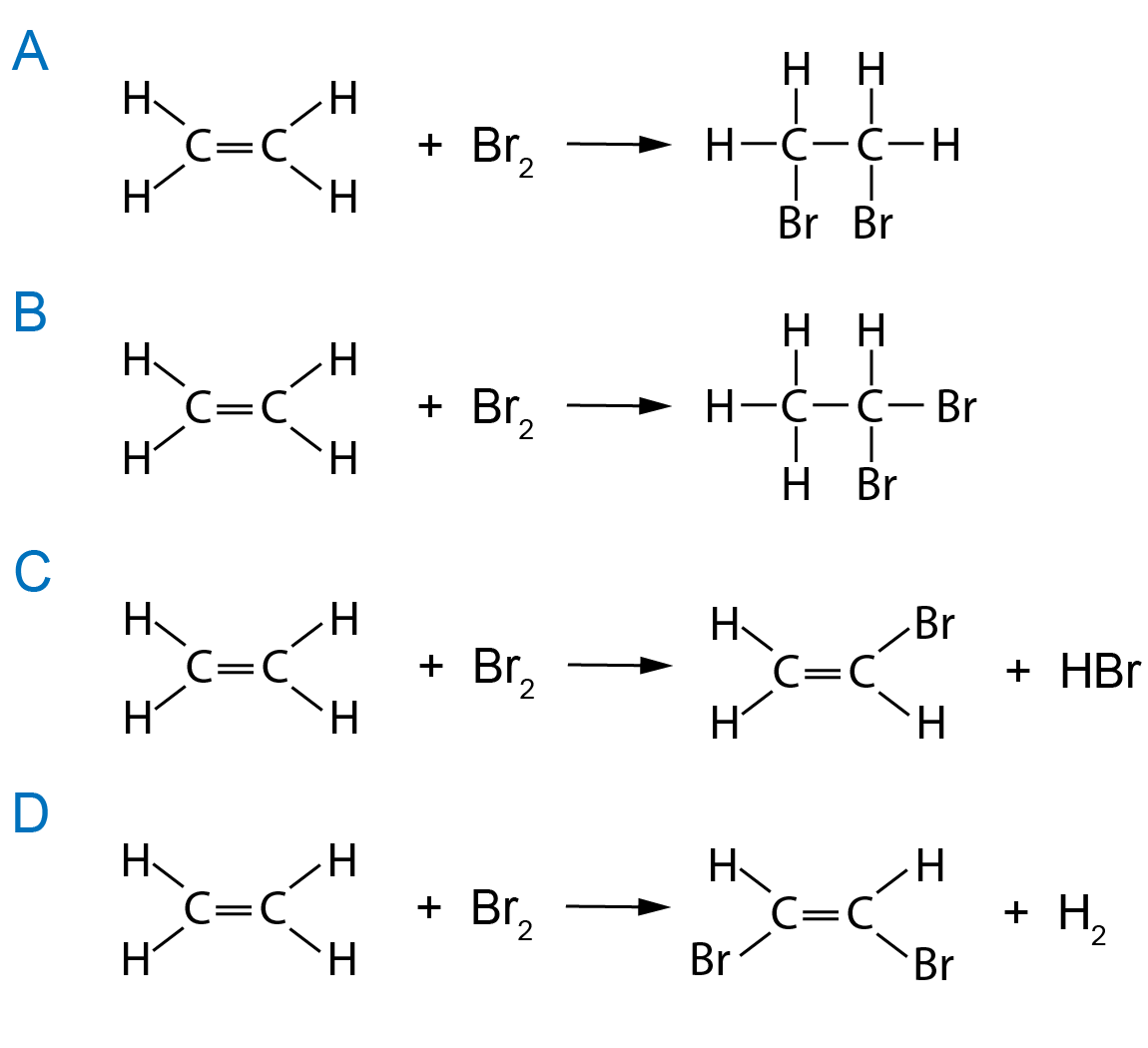Bromine Reaction . Potassium and cesium react violently with bromine. For instance, with potassium, it reacts. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. It reacts with many metals, sometimes very vigorously. Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in the presence of sodium vapor. The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. Reaction of bromine with hydrogen. Hydrogen reacts with br 2 forming hydrogen bromide. The reaction is slow at room temperature, and. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The same process works for any halogen and any alkene in which the.
from gradegorilla.com
Hydrogen reacts with br 2 forming hydrogen bromide. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Potassium and cesium react violently with bromine. For instance, with potassium, it reacts. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in the presence of sodium vapor. The same process works for any halogen and any alkene in which the. It reacts with many metals, sometimes very vigorously. Reaction of bromine with hydrogen. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound.
GCSE Chemistry Organic Grade Gorilla
Bromine Reaction Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in the presence of sodium vapor. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. For instance, with potassium, it reacts. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. It reacts with many metals, sometimes very vigorously. The same process works for any halogen and any alkene in which the. Potassium and cesium react violently with bromine. Hydrogen reacts with br 2 forming hydrogen bromide. The reaction is slow at room temperature, and. Reaction of bromine with hydrogen. The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane.
From www.coursehero.com
[Solved] The reaction between bromine and ethane occurs in the presence Bromine Reaction Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in the presence of sodium vapor. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane.. Bromine Reaction.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromine Reaction Potassium and cesium react violently with bromine. It reacts with many metals, sometimes very vigorously. Reaction of bromine with hydrogen. Hydrogen reacts with br 2 forming hydrogen bromide. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. For instance, with potassium, it reacts. Bromine reacts with many metals. Bromine Reaction.
From www.youtube.com
Reaction of aluminum with bromine YouTube Bromine Reaction While it is less reactive than fluorine or chlorine, it is more reactive than iodine. The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. Potassium and cesium react violently with bromine. For instance, with potassium, it reacts. The same process works for any halogen and any alkene in which the. It reacts with. Bromine Reaction.
From www.chemistrystudent.com
Organic Quick Notes (revision) for A2Level ChemistryStudent Bromine Reaction The reaction is slow at room temperature, and. Reaction of bromine with hydrogen. Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in the presence of sodium vapor. The same process works for any halogen and any alkene in which the. It reacts with many metals, sometimes very vigorously. Hydrogen reacts with br 2 forming hydrogen bromide.. Bromine Reaction.
From www.slideserve.com
PPT Edexcel organic reaction mechanisms PowerPoint Presentation, free Bromine Reaction Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction is slow at room temperature, and. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. Reaction of bromine with hydrogen. Hydrogen reacts with br 2. Bromine Reaction.
From www.youtube.com
Reaction of Bromine with Sodium YouTube Bromine Reaction Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Potassium and cesium react violently with bromine. Bromine. Bromine Reaction.
From omerchemblog.blogspot.com
Omer Chemistry Blog 3.8 I can describe the reaction between alkenes Bromine Reaction While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Reaction of bromine with hydrogen. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Potassium and cesium react violently with bromine. For instance, with potassium, it reacts. The same process works for any. Bromine Reaction.
From joelgordon.photoshelter.com
Reaction of bromine with an unsaturated hydrocarbon Joel Gordon Bromine Reaction Reaction of bromine with hydrogen. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. The same process works for any halogen and any alkene in which the. The reaction between bromine and ethene. Bromine Reaction.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Bromine Reaction For instance, with potassium, it reacts. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. It reacts with many metals, sometimes very vigorously. Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in the presence of sodium vapor. The same process works for any halogen. Bromine Reaction.
From www.science-revision.co.uk
Halogenation of benzene rings Bromine Reaction For instance, with potassium, it reacts. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. It reacts with many metals, sometimes very vigorously. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Reaction of bromine with. Bromine Reaction.
From gradegorilla.com
GCSE Chemistry Organic Grade Gorilla Bromine Reaction Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Reaction of bromine with hydrogen. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in. Bromine Reaction.
From www.toppr.com
Initial attack in the reaction between 1 butene and bromine will be Bromine Reaction Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. For instance, with potassium, it reacts. Potassium and cesium react violently with bromine. Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in the presence of sodium vapor. The reaction is slow at room temperature, and.. Bromine Reaction.
From www.masterorganicchemistry.com
The HellVolhardZelinsky Reaction Master Organic Chemistry Bromine Reaction It reacts with many metals, sometimes very vigorously. The reaction is slow at room temperature, and. The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. Reaction of bromine with hydrogen. For instance, with potassium, it reacts. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being. Bromine Reaction.
From www.myxxgirl.com
Explain The Reaction Mechanism Of Addition Of Bromine On Acetylene By Bromine Reaction Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. While it. Bromine Reaction.
From fphoto.photoshelter.com
science exothermic reaction sodium bromine Fundamental Photographs Bromine Reaction The same process works for any halogen and any alkene in which the. The reaction is slow at room temperature, and. Hydrogen reacts with br 2 forming hydrogen bromide. For instance, with potassium, it reacts. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. The reaction between bromine. Bromine Reaction.
From www.youtube.com
Chemistry experiment 32 Reaction between bromine and aluminium YouTube Bromine Reaction Hydrogen reacts with br 2 forming hydrogen bromide. For instance, with potassium, it reacts. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. Reaction of bromine with hydrogen. Bromination is a chemical reaction involving the reaction of a. Bromine Reaction.
From www.masterorganicchemistry.com
Bromination of Alkenes Master Organic Chemistry Bromine Reaction Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. The same process works for any halogen and any alkene in which the. Bromination is a chemical reaction involving the reaction of a compound,. Bromine Reaction.
From brunofuga.adv.br
When Bromine Gas Reacts With Aqueous Sodium Hydroxide, The, 57 OFF Bromine Reaction While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in the presence of sodium vapor. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. Hydrogen reacts with br 2 forming. Bromine Reaction.
From saniyahghopdalton.blogspot.com
Reaction of Cyclohexene With Bromine Bromine Reaction Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in the presence of sodium vapor.. Bromine Reaction.
From www.teachoo.com
Unsaturated hydrocarbons contain multiple bonds between 2 carbon atoms Bromine Reaction While it is less reactive than fluorine or chlorine, it is more reactive than iodine. The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. Reaction of bromine with hydrogen. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. For instance, with. Bromine Reaction.
From www.doubtnut.com
Toluene reacts with bromine in the presence of the light to give benzy Bromine Reaction It reacts with many metals, sometimes very vigorously. Potassium and cesium react violently with bromine. Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in the presence of sodium vapor. Hydrogen reacts with br 2 forming hydrogen bromide. For instance, with potassium, it reacts. The reaction between bromine and ethene is an example of an addition reaction. Bromine Reaction.
From www.numerade.com
SOLVED Using condensed structure, write the balanced chemical equation Bromine Reaction Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. The same process works for any halogen and any alkene in which the. Hydrogen reacts with br 2 forming hydrogen bromide. The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. While it. Bromine Reaction.
From www.numerade.com
SOLVEDThe reaction of bromine with toluene in th… Bromine Reaction The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. It reacts with many metals, sometimes very vigorously. Reaction of bromine with hydrogen. Hydrogen reacts with br 2 forming hydrogen bromide. Bromine reacts. Bromine Reaction.
From www.youtube.com
Reaction of Aluminium with Bromine YouTube Bromine Reaction Hydrogen reacts with br 2 forming hydrogen bromide. It reacts with many metals, sometimes very vigorously. The reaction is slow at room temperature, and. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in. Bromine Reaction.
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Bromine Reaction Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in the presence of sodium vapor. For instance, with potassium, it reacts. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine. Bromine Reaction.
From www.youtube.com
Neutralization of Bromine Beautiful Reaction YouTube Bromine Reaction The reaction is slow at room temperature, and. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. Reaction of bromine with hydrogen. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. For instance, with potassium, it reacts. The same process works for. Bromine Reaction.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine Bromine Reaction Reaction of bromine with hydrogen. The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. Potassium and cesium react violently with bromine. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Hydrogen reacts with br 2 forming hydrogen bromide. Bromine reacts with. Bromine Reaction.
From www.vedantu.com
Reaction of bromine water phenol gives white ppt ofA. o bromophenolB Bromine Reaction The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. Hydrogen reacts with br 2 forming hydrogen bromide. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. While it is less reactive than fluorine or chlorine, it is more reactive than iodine.. Bromine Reaction.
From www.shutterstock.com
Reaction Bromine Propene Chemical Structure Vector Vector có sẵn (miễn Bromine Reaction The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. Potassium and cesium react violently with bromine. It reacts with many metals, sometimes very vigorously. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The same process works for any halogen and. Bromine Reaction.
From www.youtube.com
Ethene + Hydrogen Bromide (Reaction and Mechanism) YouTube Bromine Reaction While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. The reaction is slow at room temperature, and.. Bromine Reaction.
From alaynameowzavala.blogspot.com
Cyclohexane Reaction With Bromine Bromine Reaction Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Bromine reacts with many metals to produce bromides, with sodium reacting vigorously in the presence of sodium vapor. Potassium and cesium react violently with. Bromine Reaction.
From www.youtube.com
Alkenes Advanced 2. Reaction of ethene with bromine YouTube Bromine Reaction The same process works for any halogen and any alkene in which the. The reaction between bromine and ethene is an example of an addition reaction and forms dibromoethane. The reaction is slow at room temperature, and. Potassium and cesium react violently with bromine. For instance, with potassium, it reacts. Alkenes react in the cold with pure liquid bromine, or. Bromine Reaction.
From www.coursehero.com
[Solved] When benzene reacts with bromine gas, the bromine atom Bromine Reaction Reaction of bromine with hydrogen. It reacts with many metals, sometimes very vigorously. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. The reaction is slow at room temperature, and. The same process. Bromine Reaction.
From www.sciencephoto.com
Bromine reaction Stock Image A500/0458 Science Photo Library Bromine Reaction For instance, with potassium, it reacts. The same process works for any halogen and any alkene in which the. Potassium and cesium react violently with bromine. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Reaction of bromine with hydrogen. Bromine reacts with many metals to produce bromides,. Bromine Reaction.
From www.youtube.com
SODIUM Metal and Bromine Reaction (There's an EXPLOSION!) YouTube Bromine Reaction For instance, with potassium, it reacts. While it is less reactive than fluorine or chlorine, it is more reactive than iodine. The reaction is slow at room temperature, and. Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. Hydrogen reacts with br 2 forming hydrogen bromide. Alkenes react. Bromine Reaction.