Qualification Method . Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. Multivariate statistical experimental design can be. Method qualification is voluntary, early. Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose. Learn how to complete a usmca form for products exported to canada or mexico that originate from the u.s., canada or mexico. Purposely vary method parameters over a known range, and determining the effect (if any) on the method results. The process for pharmaceutical analytical method development, qualification, and validation involves several crucial steps and is. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements.
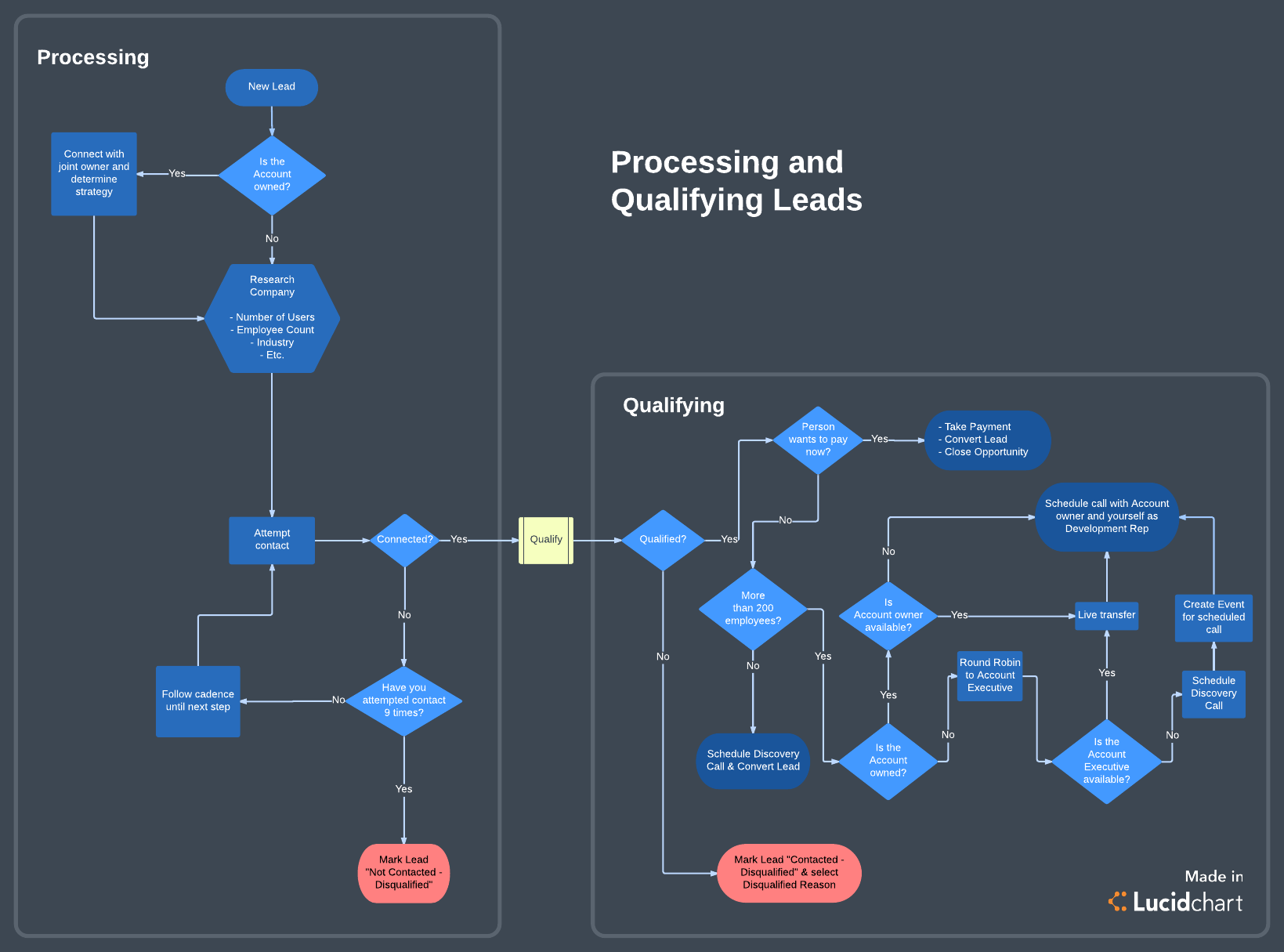
from www.lucidchart.com
The process for pharmaceutical analytical method development, qualification, and validation involves several crucial steps and is. Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. Method qualification is voluntary, early. It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements. Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. Purposely vary method parameters over a known range, and determining the effect (if any) on the method results. Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. Learn how to complete a usmca form for products exported to canada or mexico that originate from the u.s., canada or mexico. Multivariate statistical experimental design can be.
What is BANT and How Can It Streamline Lead Qualification? Lucidchart
Qualification Method Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements. Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. The process for pharmaceutical analytical method development, qualification, and validation involves several crucial steps and is. Learn how to complete a usmca form for products exported to canada or mexico that originate from the u.s., canada or mexico. Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. Method qualification is voluntary, early. Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. Multivariate statistical experimental design can be. Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose. Purposely vary method parameters over a known range, and determining the effect (if any) on the method results.
From www.lucidchart.com
What Is the MEDDIC Sales Process? Lucidchart Qualification Method Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. Multivariate statistical. Qualification Method.
From sellingsignals.com
Lead Qualification How to Qualify Leads the Right Way Selling Signals Qualification Method Learn how to complete a usmca form for products exported to canada or mexico that originate from the u.s., canada or mexico. The process for pharmaceutical analytical method development, qualification, and validation involves several crucial steps and is. Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose. Qualification. Qualification Method.
From www.lucidchart.com
What is BANT and How Can It Streamline Lead Qualification? Lucidchart Qualification Method Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose. Purposely vary method parameters over a known range, and determining the effect (if any) on the method results. Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for. Qualification Method.
From www.researchgate.net
(a) Schematic diagram of technology qualification method by DNV (b Qualification Method Multivariate statistical experimental design can be. Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. Method qualification is voluntary, early. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. Learn how to complete a usmca form for products exported to canada or mexico that originate from the u.s.,. Qualification Method.
From www.getreskilled.com
Qualification vs Validation in Pharma GetReskilled Qualification Method Purposely vary method parameters over a known range, and determining the effect (if any) on the method results. Method qualification is voluntary, early. It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements. Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended. Qualification Method.
From www.structurely.com
5 Lead Qualification Methods to Streamline Sales Qualification Method It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements. Learn how to complete a usmca form for products exported to canada or mexico that originate from the u.s., canada or mexico. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. Qualification is the process of verifying. Qualification Method.
From revnew.com
Lead Qualification Methods 7 Methodology for Sales Success Qualification Method Method qualification is voluntary, early. Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. Purposely vary method parameters over a known range, and determining the effect (if any) on the. Qualification Method.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Qualification Method Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. Purposely vary method parameters over a known range, and determining the effect (if any) on the method results. Multivariate statistical experimental design can be. Learn how to complete a usmca. Qualification Method.
From asm.aviyaan.com
Step 2 Appointment of Feasibility Study Consultant Qualification Method Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. The process for pharmaceutical analytical method development, qualification, and validation involves several crucial steps and is. Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose. Analytical method development (amd). Qualification Method.
From www.linkedin.com
Mastering Lead Qualification Your Blueprint for Sales Success Qualification Method It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements. Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. The process for pharmaceutical analytical method development, qualification, and validation involves several crucial steps and is. Analytical method development (amd) and analytical method qualification. Qualification Method.
From www.researchgate.net
Evaluation factors and qualification method while defining pressures Qualification Method Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. The process for pharmaceutical analytical method development, qualification, and validation involves. Qualification Method.
From mpl.loesungsfabrik.de
Calibration, qualification and validation in the lab what's that? Qualification Method Learn how to complete a usmca form for products exported to canada or mexico that originate from the u.s., canada or mexico. Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby. Qualification Method.
From www.studocu.com
Differences Between Validation, Calibration, Qualification DIFFERENCE Qualification Method It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements. Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. Method qualification is voluntary, early. Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose. Qualification is. Qualification Method.
From salesblink.io
How to use BANT in sales to qualify leads in 2024? Qualification Method Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. Learn how to complete a usmca form for products exported to canada or mexico that originate from the u.s., canada or mexico. Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. Qualification is the process. Qualification Method.
From www.dreamstime.com
Qualification Vector Icons on White Background Stock Vector Qualification Method Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. Multivariate statistical experimental design can be. Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. The process for pharmaceutical analytical method development, qualification, and validation involves several crucial steps and. Qualification Method.
From www.s2wmedia.com
Lead Qualification Process A Guide to Qualifying and Converting Leads Qualification Method Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. Purposely vary method parameters over a known range, and determining the effect (if any) on the method. Qualification Method.
From ascpt.onlinelibrary.wiley.com
A model qualification method for mechanistic physiological QSP models Qualification Method Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. Qualification is the process of verifying that a piece of equipment or a particular item is fit. Qualification Method.
From icon-library.com
Qualification Icon 120754 Free Icons Library Qualification Method Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose. Purposely vary method parameters over a known range, and determining the effect (if any) on the method results. The process for pharmaceutical analytical method development, qualification, and validation involves several crucial steps and is. Method qualification is voluntary, early.. Qualification Method.
From www.eventura.us
HPLC Instrument Qualification and Method Validation Eventura Qualification Method Purposely vary method parameters over a known range, and determining the effect (if any) on the method results. The process for pharmaceutical analytical method development, qualification, and validation involves several crucial steps and is. Multivariate statistical experimental design can be. Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended. Qualification Method.
From pharmagxp.com
9 Important Differences Between Qualification and Validation Pharma GxP Qualification Method Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. Method qualification is voluntary, early. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose.. Qualification Method.
From lcxyen.whuhzzs.com
A performance qualification method of blood virus nucleic acid testing Qualification Method Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. Multivariate statistical experimental design can be. Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. Method qualification is voluntary, early. Purposely vary method parameters over a known range, and determining. Qualification Method.
From sitemate.com
Procedure Qualification Record (PQR) template Digital format or PDF Qualification Method Learn how to complete a usmca form for products exported to canada or mexico that originate from the u.s., canada or mexico. It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements. Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. Analytical method development (amd) and analytical. Qualification Method.
From happsales.com
BANT Lead Qualification Modern BANT Framework Qualification Method Learn how to complete a usmca form for products exported to canada or mexico that originate from the u.s., canada or mexico. It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements. Method qualification is voluntary, early. Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. Analytical. Qualification Method.
From www.linkedin.com
Why should we apply the requalification method to employees who have Qualification Method Multivariate statistical experimental design can be. Purposely vary method parameters over a known range, and determining the effect (if any) on the method results. The process for pharmaceutical analytical method development, qualification, and validation involves several crucial steps and is. Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. Qualification. Qualification Method.
From www.shutterstock.com
Qualification Chart Icons Keywords Experience Skill Stock Vector Qualification Method The process for pharmaceutical analytical method development, qualification, and validation involves several crucial steps and is. It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements. Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. Qualification is the process of verifying that a piece of equipment or. Qualification Method.
From handypdf.com
Summary of Qualifications Sample Edit, Fill, Sign Online Handypdf Qualification Method It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose. Qualification is objective evidence that equipment or. Qualification Method.
From pronnel.com
Choosing The Best Lead Qualification Method for Your Industry Qualification Method Purposely vary method parameters over a known range, and determining the effect (if any) on the method results. The process for pharmaceutical analytical method development, qualification, and validation involves several crucial steps and is. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. It focuses on ensuring that all aspects of the equipment or. Qualification Method.
From blog.cacaomedia.co
The Ultimate Lead Qualification Method Qualification Method It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements. Purposely vary method parameters over a known range, and determining the effect (if any) on the method results. Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. Method qualification is voluntary, early. Analytical method development (amd) and. Qualification Method.
From www.researchgate.net
Qualification criteria for peer review method (part of the SME Qualification Method Purposely vary method parameters over a known range, and determining the effect (if any) on the method results. The process for pharmaceutical analytical method development, qualification, and validation involves several crucial steps and is. Learn how to complete a usmca form for products exported to canada or mexico that originate from the u.s., canada or mexico. It focuses on ensuring. Qualification Method.
From www.azom.com
Analytical Method Validation in Thermal Analysis Qualification Method Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. It focuses on ensuring that all aspects of the equipment or. Qualification Method.
From www.csss.es
How to Understand the Levels of Qualifications C3S Business School Qualification Method Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. Qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the.. Qualification Method.
From www.rcmitgroup.com
Validation and Qualification Services RCM Life Sciences & IT Qualification Method Method qualification is voluntary, early. Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. It focuses on ensuring that all aspects of the equipment or facility meet regulatory and operational requirements. Analytical method development (amd). Qualification Method.
From www.capterra.com
Is the BANT Framework Still Effective? Capterra Qualification Method Learn the difference between method qualification and method validation, two steps to check the performance of an analytical method. Learn how to complete a usmca form for products exported to canada or mexico that originate from the u.s., canada or mexico. Analytical method development (amd) and analytical method qualification (amq) are typically iterative processes whereby the. The process for pharmaceutical. Qualification Method.
From www.slideserve.com
PPT A PRESENTATION BY A.SUDHAKAR REDDY 9948214342(DEVELOPMENT OFFICER Qualification Method Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. Purposely vary method parameters over a known range, and determining the effect (if any) on the method results. Qualification is the process of verifying that a piece of equipment or a particular item is fit for. Qualification Method.
From electriciancourses4u.co.uk
Qualifications Explained How to understand different qualifications Qualification Method Purposely vary method parameters over a known range, and determining the effect (if any) on the method results. Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. Qualification primarily evaluates the fitness of equipment, systems, or models, ensuring they meet predetermined specifications. Method qualification is. Qualification Method.