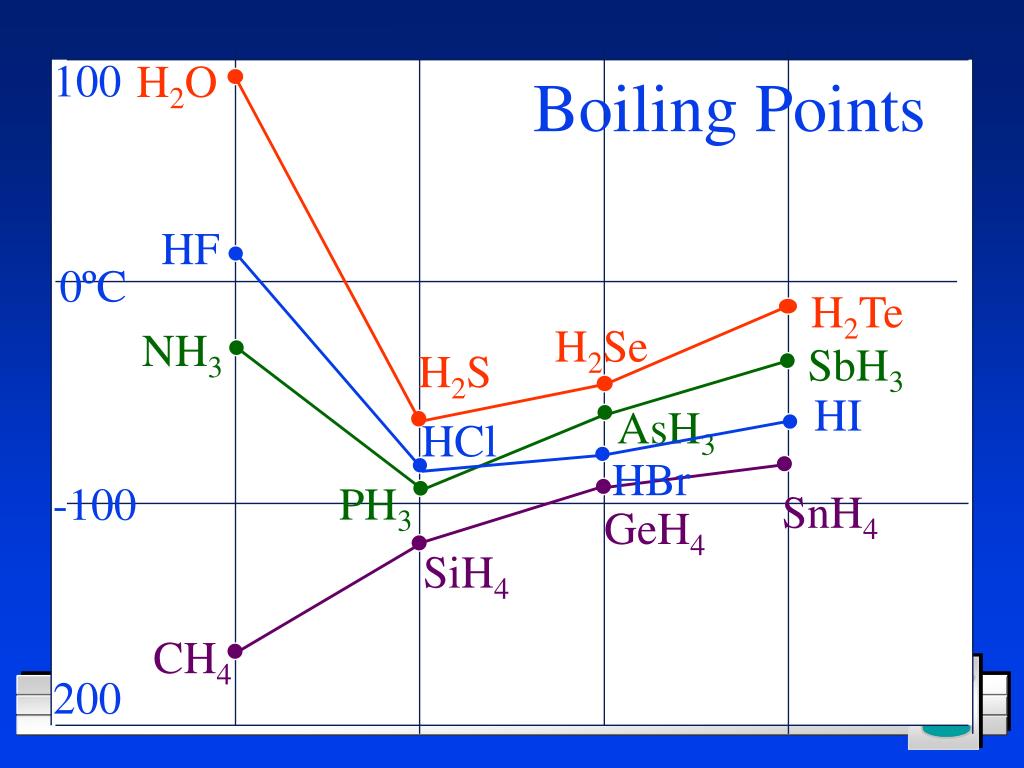
Chlorine Has A Higher Boiling Point . chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. They have the same number of electrons in their outer shell, so they are. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. Fluorine has the lowest melting and. higher melting and boiling points signify stronger noncovalent intermolecular forces. the halogens have low melting points and low boiling points. The boiling point of a substance is the temperature at which this phase change. elements in the same group of the periodic table show trends in physical properties, such as boiling point. Consider the boiling points of increasingly larger hydrocarbons.
from www.slideserve.com
the halogens have low melting points and low boiling points. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. The boiling point of a substance is the temperature at which this phase change. They have the same number of electrons in their outer shell, so they are. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. Consider the boiling points of increasingly larger hydrocarbons. higher melting and boiling points signify stronger noncovalent intermolecular forces. elements in the same group of the periodic table show trends in physical properties, such as boiling point. Fluorine has the lowest melting and.
PPT Chapter 15 PowerPoint Presentation, free download ID27506
Chlorine Has A Higher Boiling Point Consider the boiling points of increasingly larger hydrocarbons. higher melting and boiling points signify stronger noncovalent intermolecular forces. Fluorine has the lowest melting and. The boiling point of a substance is the temperature at which this phase change. They have the same number of electrons in their outer shell, so they are. the halogens have low melting points and low boiling points. elements in the same group of the periodic table show trends in physical properties, such as boiling point. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. Consider the boiling points of increasingly larger hydrocarbons. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$.
From brainly.com
Why does Sodium Chloride have a higher melting point than Sugar? a Chlorine Has A Higher Boiling Point Consider the boiling points of increasingly larger hydrocarbons. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. They have the same number of electrons in their outer shell, so they are. Fluorine has the lowest melting and. elements in the same group of the periodic table show trends in physical properties,. Chlorine Has A Higher Boiling Point.
From www.numerade.com
SOLVED Which solution will have a higher boiling point A solution Chlorine Has A Higher Boiling Point Fluorine has the lowest melting and. They have the same number of electrons in their outer shell, so they are. elements in the same group of the periodic table show trends in physical properties, such as boiling point. Consider the boiling points of increasingly larger hydrocarbons. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der. Chlorine Has A Higher Boiling Point.
From dokumen.tips
(PDF) Boiling Point Elevation Practice Wikispacesand+FP+Practice Chlorine Has A Higher Boiling Point They have the same number of electrons in their outer shell, so they are. the halogens have low melting points and low boiling points. higher melting and boiling points signify stronger noncovalent intermolecular forces. Consider the boiling points of increasingly larger hydrocarbons. Fluorine has the lowest melting and. Chlorine, cl 2, is a much smaller molecule with comparatively. Chlorine Has A Higher Boiling Point.
From www.trinityevansville.org
Melting Point Of Licl Chlorine Has A Higher Boiling Point the halogens have low melting points and low boiling points. They have the same number of electrons in their outer shell, so they are. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. elements in the same group of the periodic table show trends in physical properties, such as boiling. Chlorine Has A Higher Boiling Point.
From www.quora.com
Does chlorine have a lower boiling point than water? Quora Chlorine Has A Higher Boiling Point Consider the boiling points of increasingly larger hydrocarbons. Fluorine has the lowest melting and. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. the halogens have low melting points and low boiling points. They have the same number of electrons in their outer shell, so they are. higher. Chlorine Has A Higher Boiling Point.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID27506 Chlorine Has A Higher Boiling Point The boiling point of a substance is the temperature at which this phase change. higher melting and boiling points signify stronger noncovalent intermolecular forces. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of. Chlorine Has A Higher Boiling Point.
From www.meritnation.com
Q) Out of ethyl bromide and ethyl chloride which has higher boiling Chlorine Has A Higher Boiling Point They have the same number of electrons in their outer shell, so they are. higher melting and boiling points signify stronger noncovalent intermolecular forces. The boiling point of a substance is the temperature at which this phase change. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. Fluorine has. Chlorine Has A Higher Boiling Point.
From andreakruwwoodward.blogspot.com
The Boiling Points of Hydrogen Chloride and Hydrogen Bromide Chlorine Has A Higher Boiling Point elements in the same group of the periodic table show trends in physical properties, such as boiling point. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. Consider the boiling points of. Chlorine Has A Higher Boiling Point.
From publicaffairsworld.com
what is the boiling point of sodium chloride Chlorine Has A Higher Boiling Point Consider the boiling points of increasingly larger hydrocarbons. the halogens have low melting points and low boiling points. They have the same number of electrons in their outer shell, so they are. elements in the same group of the periodic table show trends in physical properties, such as boiling point. Chlorine, cl 2, is a much smaller molecule. Chlorine Has A Higher Boiling Point.
From cezmpyxe.blob.core.windows.net
Chlorine Higher Boiling Point Than Iodine at Garry Barton blog Chlorine Has A Higher Boiling Point Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. the halogens have low melting points and low boiling points. higher melting and boiling points signify stronger noncovalent intermolecular forces. The boiling point of a substance is the temperature at which this phase change. They have the same number. Chlorine Has A Higher Boiling Point.
From slideplayer.com
V. Solutions. ppt download Chlorine Has A Higher Boiling Point the halogens have low melting points and low boiling points. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Consider the boiling points of increasingly larger hydrocarbons. The boiling point of a substance is the temperature at which this phase change. higher melting and boiling points signify stronger noncovalent intermolecular. Chlorine Has A Higher Boiling Point.
From orlandoxitate.blogspot.com
Why Does Potassium Chloride Have a High Melting Point Chlorine Has A Higher Boiling Point Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. Consider the boiling points of increasingly larger hydrocarbons. The boiling point of a substance is the temperature at which this phase change. higher melting and boiling points signify stronger noncovalent intermolecular forces. They have the same number of electrons in. Chlorine Has A Higher Boiling Point.
From www.numerade.com
SOLVED show the formation of sodium chloride by electron draw Chlorine Has A Higher Boiling Point The boiling point of a substance is the temperature at which this phase change. the halogens have low melting points and low boiling points. elements in the same group of the periodic table show trends in physical properties, such as boiling point. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and. Chlorine Has A Higher Boiling Point.
From cexqwbtk.blob.core.windows.net
Chlorine Water Boiling Point at John Dixon blog Chlorine Has A Higher Boiling Point The boiling point of a substance is the temperature at which this phase change. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. higher melting and boiling points signify stronger noncovalent intermolecular forces. elements in the same group of the periodic table show trends in physical properties, such as boiling. Chlorine Has A Higher Boiling Point.
From www.masterorganicchemistry.com
3 Trends That Affect Boiling Points — Master Organic Chemistry Chlorine Has A Higher Boiling Point Consider the boiling points of increasingly larger hydrocarbons. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. They have the same number of electrons in their outer shell, so they are. the halogens have low melting points and low boiling points. elements in the same group of the. Chlorine Has A Higher Boiling Point.
From www.numerade.com
SOLVED Why does hydrogen chloride have a higher boiling point than Chlorine Has A Higher Boiling Point They have the same number of electrons in their outer shell, so they are. Consider the boiling points of increasingly larger hydrocarbons. Fluorine has the lowest melting and. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a. Chlorine Has A Higher Boiling Point.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Chlorine Has A Higher Boiling Point Fluorine has the lowest melting and. They have the same number of electrons in their outer shell, so they are. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Consider the boiling points of increasingly larger hydrocarbons. elements in the same group of the periodic table show trends in physical properties,. Chlorine Has A Higher Boiling Point.
From www.folkloremiperu.com
Why Does Sodium Chloride Have A High Melting Point? Perú toda la Chlorine Has A Higher Boiling Point They have the same number of electrons in their outer shell, so they are. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. Fluorine has the lowest melting and. the halogens have. Chlorine Has A Higher Boiling Point.
From www.nagwa.com
Question Video Understanding the Difference in Boiling and Freezing Chlorine Has A Higher Boiling Point the halogens have low melting points and low boiling points. Consider the boiling points of increasingly larger hydrocarbons. Fluorine has the lowest melting and. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. higher melting and boiling points signify stronger noncovalent intermolecular forces. The boiling point of a substance is. Chlorine Has A Higher Boiling Point.
From material-properties.org
Chlorine Thermal Properties Melting Point Thermal Conductivity Chlorine Has A Higher Boiling Point They have the same number of electrons in their outer shell, so they are. higher melting and boiling points signify stronger noncovalent intermolecular forces. Consider the boiling points of increasingly larger hydrocarbons. the halogens have low melting points and low boiling points. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of. Chlorine Has A Higher Boiling Point.
From www.numerade.com
SOLVED List the intermolecular forces present in hydrogen halides Chlorine Has A Higher Boiling Point Fluorine has the lowest melting and. elements in the same group of the periodic table show trends in physical properties, such as boiling point. the halogens have low melting points and low boiling points. The boiling point of a substance is the temperature at which this phase change. Chlorine, cl 2, is a much smaller molecule with comparatively. Chlorine Has A Higher Boiling Point.
From www.youtube.com
elevation in boiling point solution chapter 2 chemistry 12th Chlorine Has A Higher Boiling Point chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. the halogens have low melting points and low boiling points. higher melting and boiling points signify stronger noncovalent intermolecular forces. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. elements. Chlorine Has A Higher Boiling Point.
From wagine.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Chlorine Has A Higher Boiling Point the halogens have low melting points and low boiling points. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. elements in the same group of the periodic table show trends in physical properties, such as boiling point. The boiling point of a substance is the temperature at which this phase. Chlorine Has A Higher Boiling Point.
From www.numerade.com
SOLVEDSodium chloride has a melting point of 1074 K and a boiling Chlorine Has A Higher Boiling Point higher melting and boiling points signify stronger noncovalent intermolecular forces. They have the same number of electrons in their outer shell, so they are. Consider the boiling points of increasingly larger hydrocarbons. elements in the same group of the periodic table show trends in physical properties, such as boiling point. Fluorine has the lowest melting and. chlorine. Chlorine Has A Higher Boiling Point.
From cezmpyxe.blob.core.windows.net
Chlorine Higher Boiling Point Than Iodine at Garry Barton blog Chlorine Has A Higher Boiling Point higher melting and boiling points signify stronger noncovalent intermolecular forces. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. They have the same number of electrons in their outer shell, so they are. elements in the same group of the periodic table show trends in physical properties, such. Chlorine Has A Higher Boiling Point.
From cezmpyxe.blob.core.windows.net
Chlorine Higher Boiling Point Than Iodine at Garry Barton blog Chlorine Has A Higher Boiling Point the halogens have low melting points and low boiling points. elements in the same group of the periodic table show trends in physical properties, such as boiling point. They have the same number of electrons in their outer shell, so they are. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of. Chlorine Has A Higher Boiling Point.
From www.slideserve.com
PPT melting & boiling points PowerPoint Presentation, free download Chlorine Has A Higher Boiling Point They have the same number of electrons in their outer shell, so they are. elements in the same group of the periodic table show trends in physical properties, such as boiling point. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Chlorine, cl 2, is a much smaller molecule with comparatively. Chlorine Has A Higher Boiling Point.
From www.youtube.com
Higher boiling point practice General Chemistry 2 Chapter 12 YouTube Chlorine Has A Higher Boiling Point chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Fluorine has the lowest melting and. They have the same number of electrons in their outer shell, so they are. elements in the same group of the periodic table show trends in physical properties, such as boiling point. Chlorine, cl 2, is. Chlorine Has A Higher Boiling Point.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.42 Understand Why Compounds with Giant Ionic Chlorine Has A Higher Boiling Point higher melting and boiling points signify stronger noncovalent intermolecular forces. elements in the same group of the periodic table show trends in physical properties, such as boiling point. The boiling point of a substance is the temperature at which this phase change. Consider the boiling points of increasingly larger hydrocarbons. Chlorine, cl 2, is a much smaller molecule. Chlorine Has A Higher Boiling Point.
From cexqwbtk.blob.core.windows.net
Chlorine Water Boiling Point at John Dixon blog Chlorine Has A Higher Boiling Point They have the same number of electrons in their outer shell, so they are. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. the halogens have low melting points and low boiling points. The boiling point of a substance is the temperature at which this phase change. Chlorine, cl 2, is. Chlorine Has A Higher Boiling Point.
From studycopesettic.z21.web.core.windows.net
What Is Chlorines Melting Point Chlorine Has A Higher Boiling Point Fluorine has the lowest melting and. higher melting and boiling points signify stronger noncovalent intermolecular forces. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. the halogens have low melting points and low boiling points. They have the same number of electrons in their outer shell, so they are. . Chlorine Has A Higher Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point Practice Problems Chemistry Steps Chlorine Has A Higher Boiling Point chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. elements in the same group of the periodic table show trends in physical properties, such as boiling point. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. the halogens have low. Chlorine Has A Higher Boiling Point.
From www.youtube.com
Which Compound Has a Higher Boiling Point? Intermolecular Force Boiling Chlorine Has A Higher Boiling Point the halogens have low melting points and low boiling points. The boiling point of a substance is the temperature at which this phase change. Consider the boiling points of increasingly larger hydrocarbons. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. chlorine has a boiling point of $238~\mathrm{k}$. Chlorine Has A Higher Boiling Point.
From www.numerade.com
SOLVED 1 mole of glucose and sodium chloride were separately dissolved Chlorine Has A Higher Boiling Point Fluorine has the lowest melting and. the halogens have low melting points and low boiling points. chlorine has a boiling point of $238~\mathrm{k}$ while hydrogen chloride has a boiling point of $188~\mathrm{k}$. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. The boiling point of a substance is. Chlorine Has A Higher Boiling Point.
From cezmpyxe.blob.core.windows.net
Chlorine Higher Boiling Point Than Iodine at Garry Barton blog Chlorine Has A Higher Boiling Point the halogens have low melting points and low boiling points. Fluorine has the lowest melting and. They have the same number of electrons in their outer shell, so they are. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will. Consider the boiling points of increasingly larger hydrocarbons. The boiling. Chlorine Has A Higher Boiling Point.