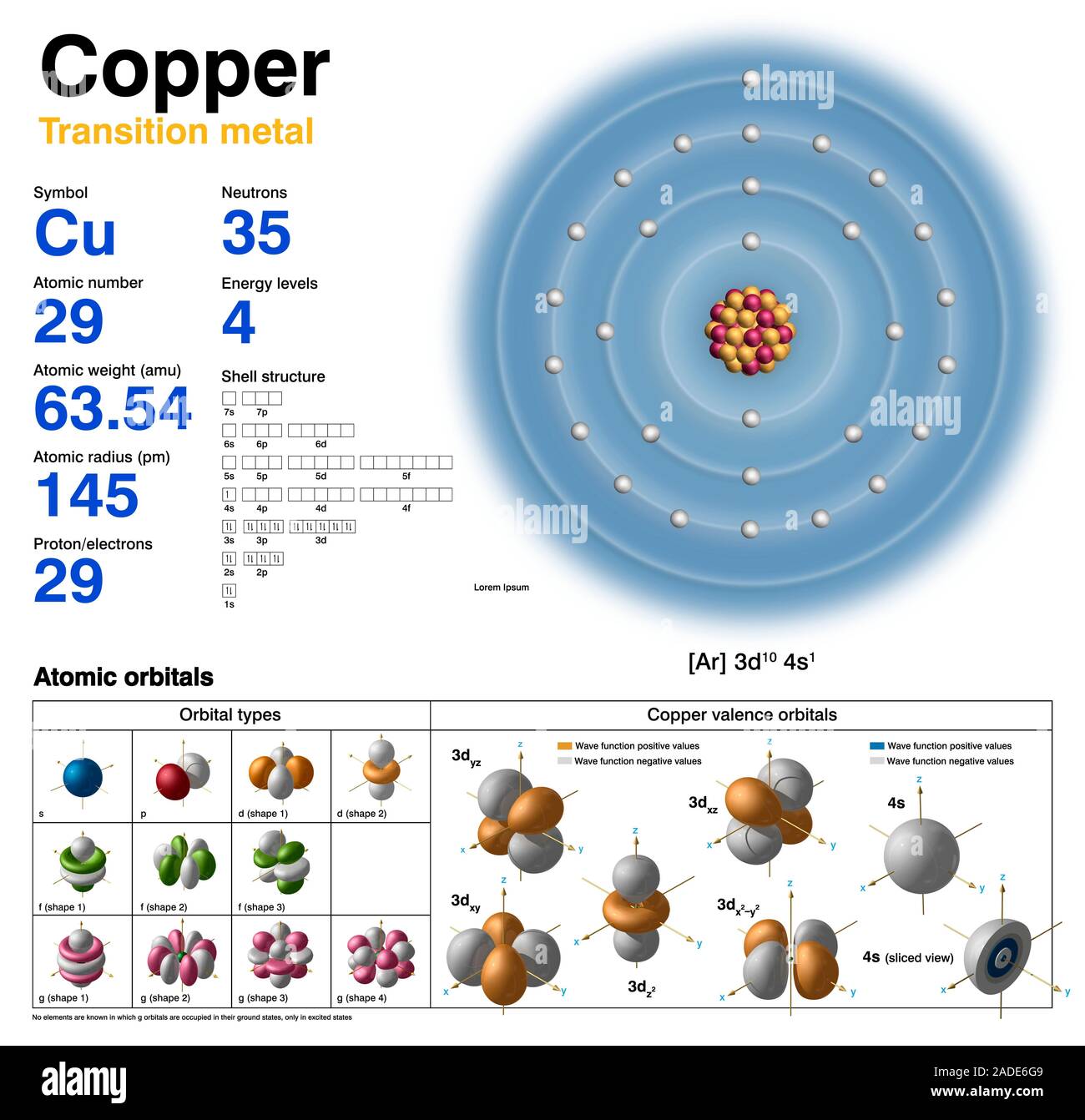
Copper Electron Configuration Class 9 . 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. Copper ion (cu +, cu 2+) electron configuration. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added stability associated 1 The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. The electron configuration for copper is: For instance, hydrogen has z = 1, and. How many inner core electrons does copper contain? The atomic number (z) is the number of protons in an atom's nucleus and defines the element. The unique feature of copper's electron configuration is that it has one electron in the 4s. The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. To write the configuration for the copper ions, first we need to write the electron configuration.
from www.alamy.com
The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. The unique feature of copper's electron configuration is that it has one electron in the 4s. How many inner core electrons does copper contain? The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. The electron configuration for copper is: To write the configuration for the copper ions, first we need to write the electron configuration. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added stability associated 1 The atomic number (z) is the number of protons in an atom's nucleus and defines the element. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. Copper ion (cu +, cu 2+) electron configuration.
Copper (Cu). Diagram of the nuclear composition, electron configuration
Copper Electron Configuration Class 9 The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. Copper ion (cu +, cu 2+) electron configuration. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. The electron configuration for copper is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. The atomic number (z) is the number of protons in an atom's nucleus and defines the element. For instance, hydrogen has z = 1, and. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added stability associated 1 The unique feature of copper's electron configuration is that it has one electron in the 4s. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. To write the configuration for the copper ions, first we need to write the electron configuration. How many inner core electrons does copper contain?
From organicful44.blogspot.com
copper orbital diagram Organicful Copper Electron Configuration Class 9 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. The atomic number (z) is the number of protons in an atom's nucleus and defines the element. To write the configuration for the copper ions, first we need to write the electron configuration. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. The ground state electron configuration. Copper Electron Configuration Class 9.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electron Configuration Class 9 The electron configuration for copper is: The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. For instance, hydrogen has z = 1, and. The unique feature of copper's electron configuration is that it has one electron in the 4s. 1s 2 2s 2 2p 6 3 s. Copper Electron Configuration Class 9.
From www.vectorstock.com
Symbol and electron diagram for copper Royalty Free Vector Copper Electron Configuration Class 9 The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. Copper ion (cu +, cu 2+) electron configuration. How many inner core electrons does copper contain? The unique feature of copper's electron configuration is that it has one electron in the 4s.. Copper Electron Configuration Class 9.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Copper Electron Configuration Class 9 Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. For instance, hydrogen has z = 1, and. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added stability associated 1 Copper ion (cu +,. Copper Electron Configuration Class 9.
From byjus.com
what is electronic configuration of copper and iron Copper Electron Configuration Class 9 How many inner core electrons does copper contain? For instance, hydrogen has z = 1, and. To write the configuration for the copper ions, first we need to write the electron configuration. The atomic number (z) is the number of protons in an atom's nucleus and defines the element. 1s 2 2s 2 2p 6 3 s 2 3p 6. Copper Electron Configuration Class 9.
From www.toppr.com
The electronic configuration of copper (29Cu) is. Copper Electron Configuration Class 9 The atomic number (z) is the number of protons in an atom's nucleus and defines the element. The electron configuration for copper is: Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. The unique feature of copper's electron configuration is that it has one electron in the 4s. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹. Copper Electron Configuration Class 9.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Class 9 The atomic number (z) is the number of protons in an atom's nucleus and defines the element. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added stability associated 1 The electron configuration for c u is 1 s 2. Copper Electron Configuration Class 9.
From ar.inspiredpencil.com
Electron Configuration Of Copper Copper Electron Configuration Class 9 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. How many inner. Copper Electron Configuration Class 9.
From www.earthdate.org
Copper’s Superpower EarthDate Copper Electron Configuration Class 9 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. For instance, hydrogen has z = 1, and. Copper ion (cu +, cu 2+) electron configuration. How many inner core electrons does copper contain? The atomic number (z) is the number of protons in an atom's nucleus and defines the element. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order. Copper Electron Configuration Class 9.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science Copper Electron Configuration Class 9 Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4. Copper Electron Configuration Class 9.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electron Configuration Class 9 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added stability associated 1 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. Copper Electron Configuration Class 9.
From edurev.in
Electronic configuration of First 20 elements.? EduRev Class 9 Question Copper Electron Configuration Class 9 The unique feature of copper's electron configuration is that it has one electron in the 4s. How many inner core electrons does copper contain? To write the configuration for the copper ions, first we need to write the electron configuration. The electron configuration for copper is: The atomic number (z) is the number of protons in an atom's nucleus and. Copper Electron Configuration Class 9.
From byjus.com
what is electronic configuration of copper and iron Copper Electron Configuration Class 9 The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. How many inner core electrons does copper contain? 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added. Copper Electron Configuration Class 9.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 29 worksheet Copper Electron Configuration Class 9 The atomic number (z) is the number of protons in an atom's nucleus and defines the element. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. To write the configuration for the copper ions, first we need to write the electron configuration. 1s 2 2s 2 2p. Copper Electron Configuration Class 9.
From www.dreamstime.com
Electron of the Element Copper Stock Vector Illustration of Copper Electron Configuration Class 9 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added stability associated 1 How many inner core electrons does copper contain? The electron configuration for copper is: To write the configuration for the copper ions, first we need to write. Copper Electron Configuration Class 9.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Class 9 The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. How many inner core electrons does copper contain? Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. The electron configuration for copper is: To write the configuration for the copper ions, first. Copper Electron Configuration Class 9.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper Electron Configuration Class 9 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added stability associated 1 The atomic number (z) is the number of protons in an atom's nucleus and defines the element. The unique feature of copper's electron configuration is that it. Copper Electron Configuration Class 9.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration Copper Electron Configuration Class 9 The unique feature of copper's electron configuration is that it has one electron in the 4s. The atomic number (z) is the number of protons in an atom's nucleus and defines the element. To write the configuration for the copper ions, first we need to write the electron configuration. How many inner core electrons does copper contain? Copper’s electron configuration. Copper Electron Configuration Class 9.
From www.slideshare.net
Copper Copper Electron Configuration Class 9 How many inner core electrons does copper contain? The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be. Copper Electron Configuration Class 9.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Electron Configuration Class 9 For instance, hydrogen has z = 1, and. The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. To write the configuration for the copper ions, first we need to write the electron configuration. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. The. Copper Electron Configuration Class 9.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes Copper Electron Configuration Class 9 How many inner core electrons does copper contain? 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added stability associated 1 For instance, hydrogen has z = 1, and. The ground state electron configuration of copper is 1s 2 2s. Copper Electron Configuration Class 9.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Class 9 The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. The atomic number (z) is the number of protons in. Copper Electron Configuration Class 9.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Electron Configuration Class 9 The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. The electron configuration for copper is: Copper ion (cu +, cu 2+) electron configuration. 1s 2 2s 2 2p 6 3 s 2. Copper Electron Configuration Class 9.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition and electron Copper Electron Configuration Class 9 The unique feature of copper's electron configuration is that it has one electron in the 4s. How many inner core electrons does copper contain? The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. 1s. Copper Electron Configuration Class 9.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Class 9 The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. Copper ion (cu +, cu 2+) electron configuration. To write the configuration for the copper ions, first we need to write the electron configuration. The electron configuration for c u is 1 s 2 2 s 2 2. Copper Electron Configuration Class 9.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Electron Configuration Class 9 Copper ion (cu +, cu 2+) electron configuration. The unique feature of copper's electron configuration is that it has one electron in the 4s. The electron configuration for copper is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. The atomic number (z) is the number of protons in an atom's nucleus and defines the element. The electron configuration for c u. Copper Electron Configuration Class 9.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper Electron Configuration Class 9 The electron configuration for copper is: How many inner core electrons does copper contain? The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. To write the configuration for the copper ions, first we need to write the electron configuration. The ground. Copper Electron Configuration Class 9.
From izayahmeowcrosby.blogspot.com
Electronic Configuration of Copper Copper Electron Configuration Class 9 The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. How many inner core electrons does copper contain? Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Copper ion (cu +, cu 2+) electron configuration. 1s². Copper Electron Configuration Class 9.
From byjus.com
Electronic Configuration of Elements Definition, Electronic Copper Electron Configuration Class 9 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. The electron configuration for copper is: 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added stability associated. Copper Electron Configuration Class 9.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Electron Configuration Class 9 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. To write the configuration for the copper ions, first we need to write the electron configuration. For instance, hydrogen has z = 1, and. The unique feature of copper's electron configuration is that it has one electron in the 4s. The electron configuration for copper is: The electron configuration for c u is. Copper Electron Configuration Class 9.
From www.youtube.com
ELECTRONIC CONFIGURATION OF COPPER ATOM and STABILITY YouTube Copper Electron Configuration Class 9 For instance, hydrogen has z = 1, and. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added stability associated 1 Copper ion (cu +,. Copper Electron Configuration Class 9.
From pushixy.blogspot.com
Electronic Configuration Of Copper copper Uses, Properties, & Facts Copper Electron Configuration Class 9 The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Copper ion (cu +, cu 2+) electron configuration. The atomic number (z) is the number of protons. Copper Electron Configuration Class 9.
From www.toppr.com
Write the electronic configuration of Cu(At No = 29),Cr(at no 24) and O^2 Copper Electron Configuration Class 9 For instance, hydrogen has z = 1, and. Copper ion (cu +, cu 2+) electron configuration. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 the actual electron configuration of these elements may be rationalized in terms of an added stability associated 1 The ground state electron configuration of copper is 1s 2 2s. Copper Electron Configuration Class 9.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Copper Electron Configuration Class 9 Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. How many inner core electrons does copper contain? For instance, hydrogen has z = 1, and. The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. The. Copper Electron Configuration Class 9.
From byjus.com
26. Why there is exception while writing the electronic configuration Copper Electron Configuration Class 9 How many inner core electrons does copper contain? The electron configuration for c u is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. The atomic number (z) is the number of protons. Copper Electron Configuration Class 9.