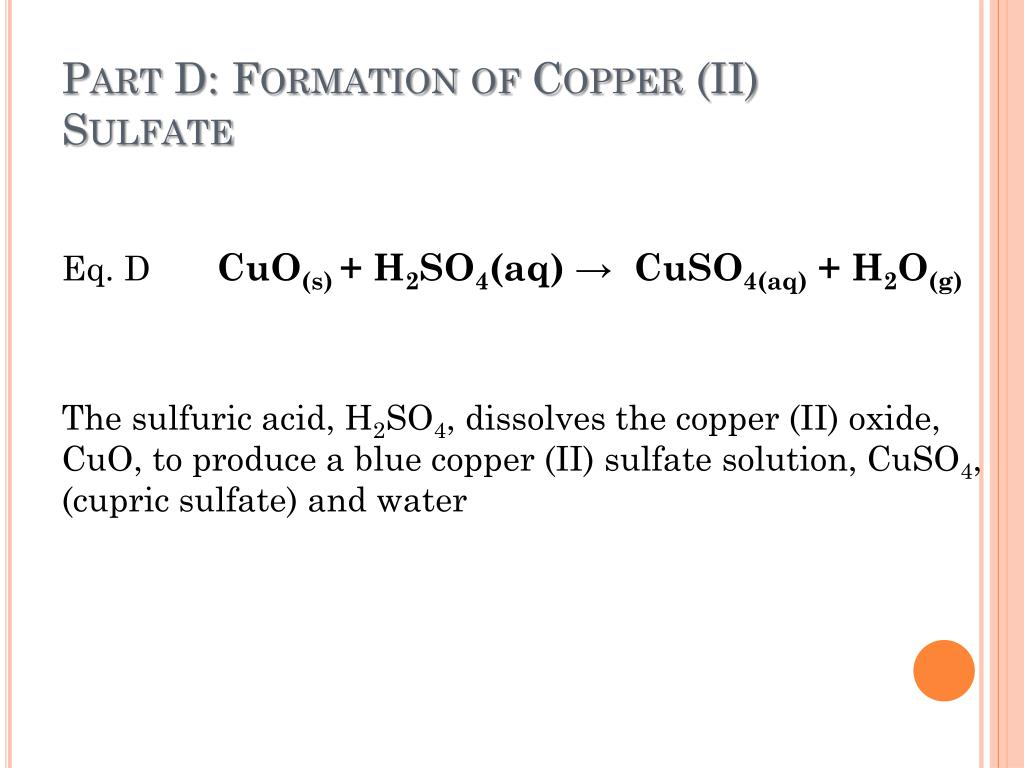
Copper Hydroxide To Copper Oxide Balanced Equation . The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. The balanced equation will appear. Copper hydroxide gives rise to oxide through the formation of a complex anion, cu(oh) 4 2−, by a reconstructive transformation. Heating copper hydroxide produces copper oxide, cuo, a black solid. Write a balanced chemical equation for the reaction that occurs when copper (ii) hydroxide decomposes into copper (ii) oxide and water. When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. How would you write a balanced equation for the reaction?
from www.slideserve.com
When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. The balanced equation will appear. Write a balanced chemical equation for the reaction that occurs when copper (ii) hydroxide decomposes into copper (ii) oxide and water. Heating copper hydroxide produces copper oxide, cuo, a black solid. How would you write a balanced equation for the reaction? Copper hydroxide gives rise to oxide through the formation of a complex anion, cu(oh) 4 2−, by a reconstructive transformation. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one.
PPT Chemical Reactions Copper Reactions PowerPoint Presentation
Copper Hydroxide To Copper Oxide Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Copper hydroxide gives rise to oxide through the formation of a complex anion, cu(oh) 4 2−, by a reconstructive transformation. Write a balanced chemical equation for the reaction that occurs when copper (ii) hydroxide decomposes into copper (ii) oxide and water. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. Heating copper hydroxide produces copper oxide, cuo, a black solid. The balanced equation will appear. When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. How would you write a balanced equation for the reaction? The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper.
From www.youtube.com
How to write the equation for CuO + H2O Copper (II) oxide + Water Copper Hydroxide To Copper Oxide Balanced Equation The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Heating copper hydroxide produces copper oxide, cuo, a black solid. The balanced equation will appear. When #cu(oh)_2#is heated, copper(ii) oxide. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation ID5482147 Copper Hydroxide To Copper Oxide Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. How would you write a balanced equation for the reaction? The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. Write. Copper Hydroxide To Copper Oxide Balanced Equation.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Copper Hydroxide To Copper Oxide Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Heating copper hydroxide produces copper oxide, cuo, a black solid. When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. Write a balanced chemical equation for the reaction that occurs when copper (ii) hydroxide decomposes into copper (ii). Copper Hydroxide To Copper Oxide Balanced Equation.
From www.numerade.com
SOLVEDExample Write Out the balanced chemical equation for the Copper Hydroxide To Copper Oxide Balanced Equation Write a balanced chemical equation for the reaction that occurs when copper (ii) hydroxide decomposes into copper (ii) oxide and water. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. When #cu(oh)_2#is heated, copper(ii) oxide. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.youtube.com
How to Balance CuO + H2 = Cu + H2O Copper (II) Oxide and Hydrogen Gas Copper Hydroxide To Copper Oxide Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.numerade.com
SOLVED Provide formulas, and a balanced equation for the following Copper Hydroxide To Copper Oxide Balanced Equation When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Copper hydroxide gives rise to oxide through the formation of a. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.chegg.com
ii. Give the balanced equation for the conversion of Copper Hydroxide To Copper Oxide Balanced Equation How would you write a balanced equation for the reaction? To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Heating copper hydroxide produces copper oxide, cuo, a black solid. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. The equation above indicates that one. Copper Hydroxide To Copper Oxide Balanced Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Hydroxide To Copper Oxide Balanced Equation Copper hydroxide gives rise to oxide through the formation of a complex anion, cu(oh) 4 2−, by a reconstructive transformation. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. Write. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.chegg.com
Solved Correct PartC olid copper (II) oxide reacts with Copper Hydroxide To Copper Oxide Balanced Equation The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Write a balanced chemical equation for the reaction that occurs when copper (ii) hydroxide decomposes into copper (ii) oxide and water. The balanced equation will appear. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.chegg.com
Solved Write A Balanced Chemical Equation For Each Reacti... Copper Hydroxide To Copper Oxide Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The balanced equation will appear. Copper hydroxide gives rise to oxide through the formation of a complex anion, cu(oh) 4 2−, by a reconstructive transformation. How would you write a balanced equation for the reaction? The equation. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.numerade.com
SOLVED write Balanced equation for copper metal and oxygen gas and Copper Hydroxide To Copper Oxide Balanced Equation When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. The balanced equation will appear. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Heating copper hydroxide produces copper oxide, cuo, a black solid. How would you write a balanced equation for the reaction? Write a balanced. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Copper Hydroxide To Copper Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. Copper hydroxide gives rise to oxide through the formation of a complex anion, cu(oh) 4 2−, by a reconstructive transformation. Heating. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.numerade.com
SOLVED 6. Copper(II) oxide reacts with hydrochloric acid to produce Copper Hydroxide To Copper Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. Copper hydroxide gives rise to oxide through the formation of a complex anion, cu(oh) 4 2−, by a reconstructive transformation. The. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.chegg.com
Solved 1 Reaction C Copper(II) Hydroxide to Copper(IT) Copper Hydroxide To Copper Oxide Balanced Equation The balanced equation will appear. Copper hydroxide gives rise to oxide through the formation of a complex anion, cu(oh) 4 2−, by a reconstructive transformation. Write a balanced chemical equation for the reaction that occurs when copper (ii) hydroxide decomposes into copper (ii) oxide and water. When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. The chemical equation described in. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.youtube.com
3. Copper Hydroxide to Copper Oxide (Cu Again Lab) YouTube Copper Hydroxide To Copper Oxide Balanced Equation Write a balanced chemical equation for the reaction that occurs when copper (ii) hydroxide decomposes into copper (ii) oxide and water. The balanced equation will appear. When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. Heating copper hydroxide produces copper oxide, cuo, a black solid. The equation above indicates that one mole of solid copper is reacting with two moles. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.youtube.com
CuO+HCl=CuCl2+H2O Balanced EquationCopper oxide+Hydrochloric acid Copper Hydroxide To Copper Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. Copper hydroxide gives rise to oxide through the formation of a complex anion, cu(oh) 4 2−, by a reconstructive transformation. How would you write a balanced equation for the reaction? The balanced equation will. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.chegg.com
Solved Write a balanced chemical equation showing the Copper Hydroxide To Copper Oxide Balanced Equation The balanced equation will appear. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. Heating copper hydroxide produces copper oxide, cuo, a black solid. When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. Copper hydroxide gives rise to oxide through the formation of a complex anion, cu(oh). Copper Hydroxide To Copper Oxide Balanced Equation.
From link.springer.com
Copper(II) oxide nanocatalyst preparation and characterization green Copper Hydroxide To Copper Oxide Balanced Equation When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. Write a balanced chemical equation for the reaction that occurs when copper (ii) hydroxide decomposes into copper (ii) oxide and water. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. To balance a chemical equation, enter an equation. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.coursehero.com
[Solved] Cycle Step 3 Heating converts the copper hydroxide to copper Copper Hydroxide To Copper Oxide Balanced Equation The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.slideserve.com
PPT Copper Functioning as an Oxygen Carrier in Chemical Looping Copper Hydroxide To Copper Oxide Balanced Equation The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. Heating copper hydroxide produces copper oxide, cuo, a black solid. The balanced equation will appear. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.numerade.com
SOLVED Consider the reaction shown. copper + oxygen → copper (I) oxide Copper Hydroxide To Copper Oxide Balanced Equation The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.chegg.com
Solved 9. Write the molecular balanced equation for the Copper Hydroxide To Copper Oxide Balanced Equation Write a balanced chemical equation for the reaction that occurs when copper (ii) hydroxide decomposes into copper (ii) oxide and water. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. To balance a chemical equation, enter an. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Copper Hydroxide To Copper Oxide Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Write a balanced chemical equation for the reaction that occurs when copper (ii) hydroxide decomposes into copper (ii) oxide and water. The equation above indicates that one mole of solid copper is reacting with two moles of. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Cu(OH)2 = CuO + H2O YouTube Copper Hydroxide To Copper Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. Heating copper hydroxide produces copper oxide, cuo, a black solid. How would. Copper Hydroxide To Copper Oxide Balanced Equation.
From brainly.in
When Cu(OH)2is heated, copper(II) oxide and water are formed. How would Copper Hydroxide To Copper Oxide Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. The equation above indicates that one mole of solid copper is. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper Hydroxide To Copper Oxide Balanced Equation The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. How would you write a balanced equation for the reaction? The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Heating copper hydroxide produces copper. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide To Copper Oxide Balanced Equation Heating copper hydroxide produces copper oxide, cuo, a black solid. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. How would you write a balanced equation for the reaction? The equation above indicates that one mole of solid copper is reacting with two moles of aqueous. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.toppr.com
Write balanced chemical equations for the following word equation Copper Hydroxide To Copper Oxide Balanced Equation Copper hydroxide gives rise to oxide through the formation of a complex anion, cu(oh) 4 2−, by a reconstructive transformation. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The balanced equation will appear. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.chegg.com
iii. Give the balanced equation for the conversion of Copper Hydroxide To Copper Oxide Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Write a balanced chemical equation for the reaction that occurs when copper (ii) hydroxide decomposes into copper (ii) oxide and water. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Copper Hydroxide To Copper Oxide Balanced Equation When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. How would you write a balanced. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Hydroxide To Copper Oxide Balanced Equation When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. Heating copper hydroxide produces copper oxide, cuo, a black solid. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.youtube.com
of Copper(II) Carbonate Hydroxide Hydrate in LateNiteLabs Copper Hydroxide To Copper Oxide Balanced Equation The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Heating copper hydroxide produces copper oxide, cuo, a black solid. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. Write a balanced chemical equation for the reaction that occurs when. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.numerade.com
SOLVED 12.0 g of copper is concerted to copper (II) oxide by heating Copper Hydroxide To Copper Oxide Balanced Equation How would you write a balanced equation for the reaction? Copper hydroxide gives rise to oxide through the formation of a complex anion, cu(oh) 4 2−, by a reconstructive transformation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Write a balanced chemical equation for the reaction that occurs when copper (ii). Copper Hydroxide To Copper Oxide Balanced Equation.
From www.youtube.com
Type of Reaction for Cu(OH)2 = CuO + H2O YouTube Copper Hydroxide To Copper Oxide Balanced Equation Copper hydroxide gives rise to oxide through the formation of a complex anion, cu(oh) 4 2−, by a reconstructive transformation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are.. Copper Hydroxide To Copper Oxide Balanced Equation.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper Hydroxide To Copper Oxide Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. When #cu(oh)_2#is heated, copper(ii) oxide and water are formed. How would you write a balanced. Copper Hydroxide To Copper Oxide Balanced Equation.