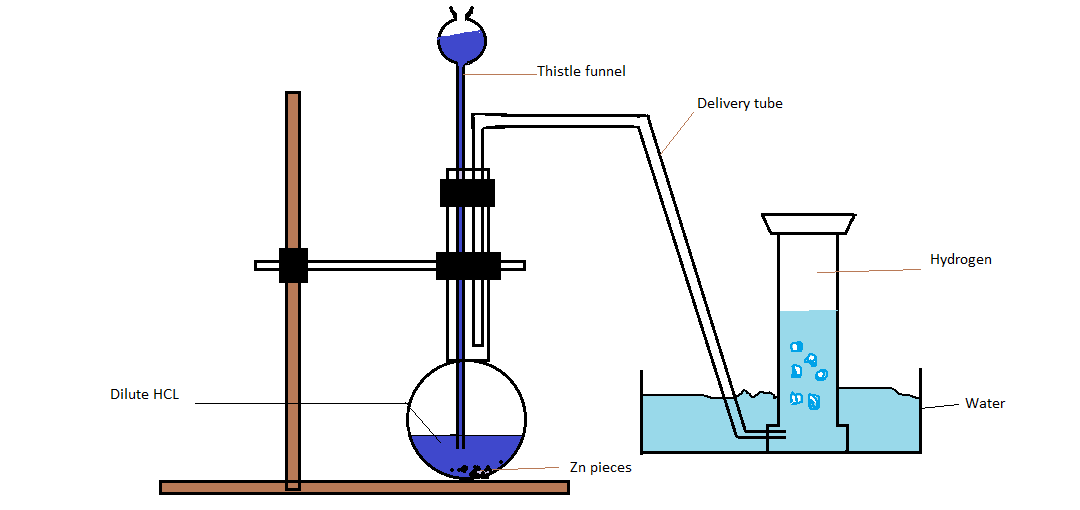
Lab Preparation Of Dry Hydrogen Gas . Metal + dilute acid → salt of metal & acid + hydrogen Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: Four main stages involved when preparing a gas in the laboratory. Here the reaction occurs in the reaction. Hydrogen can be collected over water (by passing it through water) with almost. The setup for the laboratory preparation of hydrogen gas is illustrated below. 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. Learn how to prepare hydrogen gas by reacting zinc with hydrochloric acid or by reducing metal oxides at high temperature. Gas preparation and collection methods revision notes.
from www.vedantu.com
Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: Gas preparation and collection methods revision notes. The setup for the laboratory preparation of hydrogen gas is illustrated below. Here the reaction occurs in the reaction. 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. Metal + dilute acid → salt of metal & acid + hydrogen Hydrogen can be collected over water (by passing it through water) with almost. The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. Four main stages involved when preparing a gas in the laboratory.
Explain the method of preparation of hydrogen gas in the laboratory
Lab Preparation Of Dry Hydrogen Gas Hydrogen can be collected over water (by passing it through water) with almost. Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: The setup for the laboratory preparation of hydrogen gas is illustrated below. Learn how to prepare hydrogen gas by reacting zinc with hydrochloric acid or by reducing metal oxides at high temperature. Gas preparation and collection methods revision notes. The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. Metal + dilute acid → salt of metal & acid + hydrogen This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. Here the reaction occurs in the reaction. Four main stages involved when preparing a gas in the laboratory. 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. Hydrogen can be collected over water (by passing it through water) with almost.
From www.youtube.com
Laboratory Preparation of Hydrogen Gas YouTube Lab Preparation Of Dry Hydrogen Gas This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. Four main stages involved when preparing a gas in the laboratory. Hydrogen can be collected over water (by passing it through water) with almost. Here the reaction occurs in the reaction. 2.9.5 describe the laboratory preparation and collection. Lab Preparation Of Dry Hydrogen Gas.
From www.vrogue.co
Hydrogen Gas Hydrogen Gas Lab Zinc And Hydrochloric A vrogue.co Lab Preparation Of Dry Hydrogen Gas The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. Hydrogen can be collected over water (by passing it through water) with almost. Gas preparation and collection methods revision notes. Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: Here the reaction occurs in the reaction.. Lab Preparation Of Dry Hydrogen Gas.
From www.youtube.com
Gases The preparation of Hydrogen gas (H2) in the laboratory / part Lab Preparation Of Dry Hydrogen Gas Gas preparation and collection methods revision notes. Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: Hydrogen can be collected over water (by passing it through water) with almost. Here the reaction occurs in the reaction. The setup for the laboratory preparation of hydrogen gas is illustrated below. Learn how to prepare hydrogen gas. Lab Preparation Of Dry Hydrogen Gas.
From scienceinfo.com
Preparation of Hydrogen Lab Preparation Of Dry Hydrogen Gas Metal + dilute acid → salt of metal & acid + hydrogen Learn how to prepare hydrogen gas by reacting zinc with hydrochloric acid or by reducing metal oxides at high temperature. Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: Four main stages involved when preparing a gas in the laboratory. Gas preparation. Lab Preparation Of Dry Hydrogen Gas.
From www.youtube.com
Preparation of Hydrogen Gas in the Laboratory YouTube Lab Preparation Of Dry Hydrogen Gas The setup for the laboratory preparation of hydrogen gas is illustrated below. This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: Metal + dilute acid → salt of metal & acid +. Lab Preparation Of Dry Hydrogen Gas.
From icsechemistry16.blogspot.com
laboratory preparation of HCl gas Lab Preparation Of Dry Hydrogen Gas The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. Hydrogen can be collected over water (by passing it through water) with almost. Gas preparation and collection methods revision notes.. Lab Preparation Of Dry Hydrogen Gas.
From www.vrogue.co
Hydrogen Gas Hydrogen Gas Lab Zinc And Hydrochloric A vrogue.co Lab Preparation Of Dry Hydrogen Gas The setup for the laboratory preparation of hydrogen gas is illustrated below. Gas preparation and collection methods revision notes. Here the reaction occurs in the reaction. 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. Suggest a reason why zinc is a suitable choice for. Lab Preparation Of Dry Hydrogen Gas.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Lab Preparation Of Dry Hydrogen Gas Metal + dilute acid → salt of metal & acid + hydrogen Learn how to prepare hydrogen gas by reacting zinc with hydrochloric acid or by reducing metal oxides at high temperature. This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. Four main stages involved when preparing. Lab Preparation Of Dry Hydrogen Gas.
From www.youtube.com
Lab.preparation of hydrogen gas YouTube Lab Preparation Of Dry Hydrogen Gas Here the reaction occurs in the reaction. Hydrogen can be collected over water (by passing it through water) with almost. Four main stages involved when preparing a gas in the laboratory. Gas preparation and collection methods revision notes. The setup for the laboratory preparation of hydrogen gas is illustrated below. Metal + dilute acid → salt of metal & acid. Lab Preparation Of Dry Hydrogen Gas.
From www.knowledgeboat.com
In the laboratory preparation of hydrochloric acid, hydrogen Lab Preparation Of Dry Hydrogen Gas Metal + dilute acid → salt of metal & acid + hydrogen This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. Hydrogen can be collected over water (by passing it through water) with almost. The chemical reactions that take place during the preparation of hydrogen gas via. Lab Preparation Of Dry Hydrogen Gas.
From www.youtube.com
Lab Preparation of Hydrogen (नेपाली) YouTube Lab Preparation Of Dry Hydrogen Gas Gas preparation and collection methods revision notes. Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. Four main stages involved when preparing a gas in the laboratory. Hydrogen can be. Lab Preparation Of Dry Hydrogen Gas.
From byjus.com
How is Carbon dioxide prepared in a laboratory? Lab Preparation Of Dry Hydrogen Gas This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. The setup for the laboratory preparation of hydrogen gas is illustrated below. 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. Metal +. Lab Preparation Of Dry Hydrogen Gas.
From www.alamy.com
Labelled diagram for laboratory preparation of hydrogen from zinc and Lab Preparation Of Dry Hydrogen Gas This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. Metal + dilute acid → salt of metal & acid + hydrogen Hydrogen can be collected over water (by passing it through water) with almost. Four main stages involved when preparing a gas in the laboratory. The setup. Lab Preparation Of Dry Hydrogen Gas.
From brainly.in
A student set up the apparatus shown to collect a dry sample of Lab Preparation Of Dry Hydrogen Gas This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. Gas preparation and collection methods revision notes. The setup for the laboratory preparation of hydrogen gas is illustrated below. Hydrogen can be collected over water (by passing it through water) with almost. Metal + dilute acid → salt. Lab Preparation Of Dry Hydrogen Gas.
From www.youtube.com
Lab Preparation of Hydrogen YouTube Lab Preparation Of Dry Hydrogen Gas This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. Gas preparation and collection methods revision notes. Four main stages involved when preparing a gas in the laboratory. The setup for the laboratory preparation of hydrogen gas is illustrated below. Hydrogen can be collected over water (by passing. Lab Preparation Of Dry Hydrogen Gas.
From www.vedantu.com
Explain the method of preparation of hydrogen gas in the laboratory Lab Preparation Of Dry Hydrogen Gas This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. Here the reaction occurs in the reaction. Learn how to prepare hydrogen gas by reacting zinc with hydrochloric acid or. Lab Preparation Of Dry Hydrogen Gas.
From www.youtube.com
Preparation and Properties of Hydrogen Gas YouTube Lab Preparation Of Dry Hydrogen Gas The setup for the laboratory preparation of hydrogen gas is illustrated below. Metal + dilute acid → salt of metal & acid + hydrogen Four main stages involved when preparing a gas in the laboratory. Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: Hydrogen can be collected over water (by passing it through. Lab Preparation Of Dry Hydrogen Gas.
From chem.libretexts.org
4 The Properties of Oxygen Gas (Experiment) Chemistry LibreTexts Lab Preparation Of Dry Hydrogen Gas The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: Hydrogen can be. Lab Preparation Of Dry Hydrogen Gas.
From www.alamy.com
Illustration portraying the preparation of oxygen gas Stock Photo Alamy Lab Preparation Of Dry Hydrogen Gas This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. Learn how to prepare hydrogen gas by reacting zinc with hydrochloric acid or by. Lab Preparation Of Dry Hydrogen Gas.
From www.youtube.com
LABORATORY PREPARATION OF HYDROGEN GAS AND PROCEDURE YouTube Lab Preparation Of Dry Hydrogen Gas The setup for the laboratory preparation of hydrogen gas is illustrated below. Gas preparation and collection methods revision notes. This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: Four main stages involved. Lab Preparation Of Dry Hydrogen Gas.
From www.doubtnut.com
Draw a labeled diagram for the laboratory preparation of hydrogen Lab Preparation Of Dry Hydrogen Gas Four main stages involved when preparing a gas in the laboratory. Here the reaction occurs in the reaction. 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below.. Lab Preparation Of Dry Hydrogen Gas.
From www.youtube.com
Preparation of Hydrogen in Lab Science Experiment Explanation on Lab Preparation Of Dry Hydrogen Gas Hydrogen can be collected over water (by passing it through water) with almost. 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. Here the reaction occurs in. Lab Preparation Of Dry Hydrogen Gas.
From www.projectslawn.com
[Solved] In The Following Schematic Diagram For The Preparation Of Lab Preparation Of Dry Hydrogen Gas Learn how to prepare hydrogen gas by reacting zinc with hydrochloric acid or by reducing metal oxides at high temperature. Metal + dilute acid → salt of metal & acid + hydrogen Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: Gas preparation and collection methods revision notes. This page looks at how you. Lab Preparation Of Dry Hydrogen Gas.
From guidewiringungodlier.z14.web.core.windows.net
Diagram Of Laboratory Preparation Of Hydrogen Lab Preparation Of Dry Hydrogen Gas 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. Gas preparation and collection methods revision notes. The setup for the laboratory preparation of hydrogen gas is illustrated below. This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia. Lab Preparation Of Dry Hydrogen Gas.
From wanibesakc.blogspot.com
WANIBESAK 4 Cara Pembuatan Gas Hidrogen Di Dalam Laboratorium dan Pada Lab Preparation Of Dry Hydrogen Gas Metal + dilute acid → salt of metal & acid + hydrogen Gas preparation and collection methods revision notes. This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. Here the reaction occurs in the reaction. Learn how to prepare hydrogen gas by reacting zinc with hydrochloric acid. Lab Preparation Of Dry Hydrogen Gas.
From cartoondealer.com
Preparation Of Hydrogen Gas In Laboratory With The Help Of Zinc And Lab Preparation Of Dry Hydrogen Gas The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. Here the reaction occurs in the reaction. Metal + dilute acid → salt of metal & acid + hydrogen Gas preparation and collection methods revision notes. Four main stages involved when preparing a gas in the laboratory. 2.9.5 describe the laboratory preparation. Lab Preparation Of Dry Hydrogen Gas.
From www.alamy.com
Fully labelled diagram of the laboratory preparation of hydrogen Stock Lab Preparation Of Dry Hydrogen Gas The setup for the laboratory preparation of hydrogen gas is illustrated below. Here the reaction occurs in the reaction. The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. Four main stages involved when preparing a gas in the laboratory. Suggest a reason why zinc is a suitable choice for laboratory preparation. Lab Preparation Of Dry Hydrogen Gas.
From www.slideserve.com
PPT NonMetals PowerPoint Presentation, free download ID5966722 Lab Preparation Of Dry Hydrogen Gas Four main stages involved when preparing a gas in the laboratory. The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. Suggest a reason why zinc is a. Lab Preparation Of Dry Hydrogen Gas.
From www.kenyaplex.com
(i) In the space provided sketch a labeled diagram to show how hydrogen Lab Preparation Of Dry Hydrogen Gas 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. Metal + dilute acid → salt of metal & acid + hydrogen Suggest a. Lab Preparation Of Dry Hydrogen Gas.
From www.quanswer.com
Show the diagram of preparation of dry hydrogen gas ? Quanswer Lab Preparation Of Dry Hydrogen Gas Here the reaction occurs in the reaction. Metal + dilute acid → salt of metal & acid + hydrogen 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. The chemical reactions that take place during the preparation of hydrogen gas via this method are listed. Lab Preparation Of Dry Hydrogen Gas.
From www.youtube.com
Laboratory Preparation of Hydrogen gas YouTube Lab Preparation Of Dry Hydrogen Gas Metal + dilute acid → salt of metal & acid + hydrogen Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: This page looks at how you can collect and test samples of hydrogen, oxygen, carbon dioxide, ammonia and chlorine in the lab. The chemical reactions that take place during the preparation of hydrogen. Lab Preparation Of Dry Hydrogen Gas.
From www.toppr.com
Explain the method of preparation of hydrogen gas Lab Preparation Of Dry Hydrogen Gas Here the reaction occurs in the reaction. Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: Four main stages involved when preparing a gas in the laboratory. The setup for the laboratory preparation of hydrogen gas is illustrated below. Hydrogen can be collected over water (by passing it through water) with almost. 2.9.5 describe. Lab Preparation Of Dry Hydrogen Gas.
From mavink.com
Diagram Of Hydrogen Gas Test Lab Preparation Of Dry Hydrogen Gas The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. Hydrogen can be collected over water (by passing it through water) with almost. Suggest a reason why zinc is a suitable choice for laboratory preparation of hydrogen (hint: Here the reaction occurs in the reaction. 2.9.5 describe the laboratory preparation and collection. Lab Preparation Of Dry Hydrogen Gas.
From www.youtube.com
To Prepare Hydrogen Gas in Laboratory NEB Class9 YouTube Lab Preparation Of Dry Hydrogen Gas Four main stages involved when preparing a gas in the laboratory. Hydrogen can be collected over water (by passing it through water) with almost. 2.9.5 describe the laboratory preparation and collection of hydrogen using zinc (or other suitable metal) and hydrochloric acid, and recall the physical properties of. The chemical reactions that take place during the preparation of hydrogen gas. Lab Preparation Of Dry Hydrogen Gas.
From classnotes.org.in
Preparation Of Hydrogen Chemistry, Class 11, Hydrogen Lab Preparation Of Dry Hydrogen Gas Here the reaction occurs in the reaction. The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. Learn how to prepare hydrogen gas by reacting zinc with hydrochloric acid or by reducing metal oxides at high temperature. This page looks at how you can collect and test samples of hydrogen, oxygen, carbon. Lab Preparation Of Dry Hydrogen Gas.