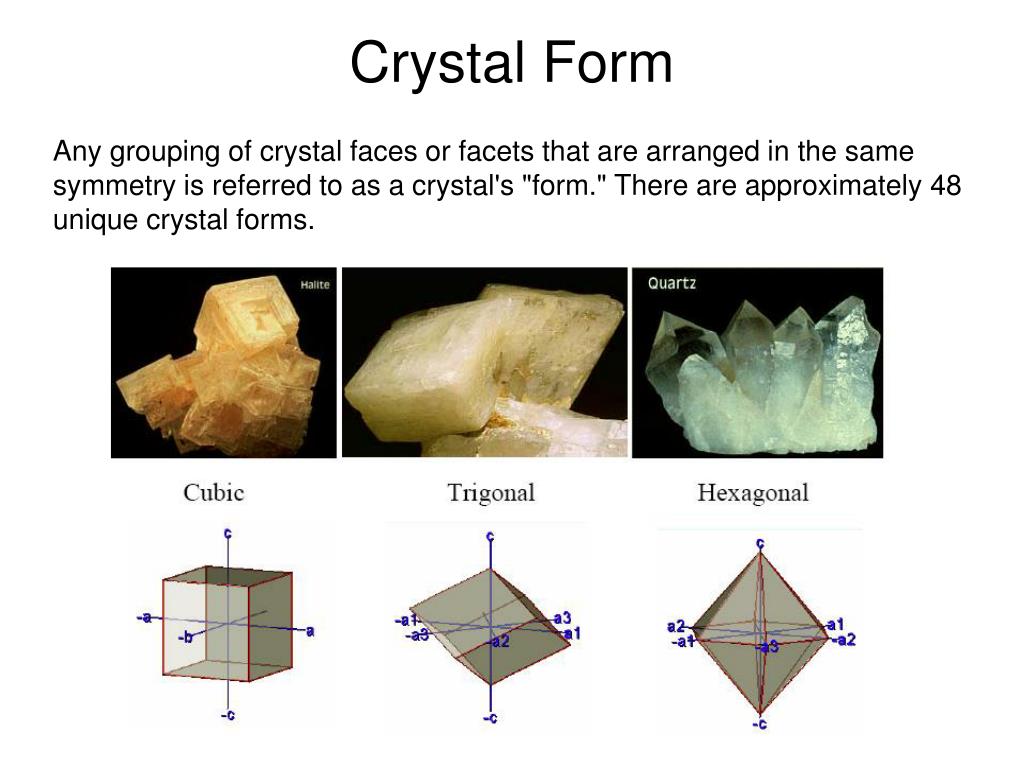
Difference Between Crystal And Non Crystal . By definition (with just a few special exceptions) minerals must be crystalline. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. This means that they are solids with an orderly repetitive atomic arrangement. Crystalline structure has atoms arranged in order whereas. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic.
from www.slideserve.com
Crystalline structure has atoms arranged in order whereas. This means that they are solids with an orderly repetitive atomic arrangement. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. By definition (with just a few special exceptions) minerals must be crystalline.
PPT Lecture 9 Rocks and Minerals PowerPoint Presentation, free
Difference Between Crystal And Non Crystal Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. By definition (with just a few special exceptions) minerals must be crystalline. This means that they are solids with an orderly repetitive atomic arrangement. Crystalline structure has atoms arranged in order whereas.
From www.pinterest.se
Tumbled and rough Crystal Healing Stones, Aventurine Crystal, Rock Difference Between Crystal And Non Crystal By definition (with just a few special exceptions) minerals must be crystalline. This means that they are solids with an orderly repetitive atomic arrangement. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Crystalline solids and noncrystalline solids are two distinct types of solid materials with. Difference Between Crystal And Non Crystal.
From www.slideserve.com
PPT Chapter 3 Matter and Energy PowerPoint Presentation, free Difference Between Crystal And Non Crystal The basic difference between crystalline and noncrystalline structures is their atomic arrangement. Crystalline structure has atoms arranged in order whereas. By definition (with just a few special exceptions) minerals must be crystalline. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. Crystal, any solid material in which the component atoms are arranged in. Difference Between Crystal And Non Crystal.
From askanydifference.com
Crystal vs Rhinestone Difference and Comparison Difference Between Crystal And Non Crystal Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. The major difference between crystalline and amorphous is crystalline. Difference Between Crystal And Non Crystal.
From www.hobbyistgeek.com
What Is The Difference Between Gemstone And Crystal? "Revealed" Difference Between Crystal And Non Crystal By definition (with just a few special exceptions) minerals must be crystalline. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. This means that they are solids with an orderly repetitive atomic arrangement. The. Difference Between Crystal And Non Crystal.
From askanydifference.com
Diamond vs Crystal Difference and Comparison Difference Between Crystal And Non Crystal By definition (with just a few special exceptions) minerals must be crystalline. Crystalline structure has atoms arranged in order whereas. This means that they are solids with an orderly repetitive atomic arrangement. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. The basic difference between crystalline. Difference Between Crystal And Non Crystal.
From www.findlight.net
XRay Diffraction Getting to Know Crystal Structures (Part Ⅰ) Difference Between Crystal And Non Crystal The basic difference between crystalline and noncrystalline structures is their atomic arrangement. Crystalline structure has atoms arranged in order whereas. This means that they are solids with an orderly repetitive atomic arrangement. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. Crystalline solids and noncrystalline solids are two distinct types of. Difference Between Crystal And Non Crystal.
From www.researchgate.net
2. These diagrams show the difference between a single crystal and a Difference Between Crystal And Non Crystal The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. By definition (with just a few special exceptions) minerals must be crystalline. Crystal, any. Difference Between Crystal And Non Crystal.
From askanydifference.com
Cristal vs Verre Différence et Comparaison Difference Between Crystal And Non Crystal Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. Crystalline structure has atoms arranged in order whereas. By definition (with just a few special exceptions) minerals must be crystalline. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. This means that they are solids with an orderly repetitive atomic arrangement.. Difference Between Crystal And Non Crystal.
From www.youtube.com
Difference between Crystalline & Non crystalline Solids SkillLync Difference Between Crystal And Non Crystal The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. By definition (with just a few special exceptions) minerals must be crystalline. This means that they are solids with an orderly repetitive atomic arrangement. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. Crystalline structure has atoms arranged in. Difference Between Crystal And Non Crystal.
From in.pinterest.com
Gemstone vs Crystal What's the Difference? in 2023 Gemstones Difference Between Crystal And Non Crystal The basic difference between crystalline and noncrystalline structures is their atomic arrangement. Crystalline structure has atoms arranged in order whereas. By definition (with just a few special exceptions) minerals must be crystalline. This means that they are solids with an orderly repetitive atomic arrangement. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid. Difference Between Crystal And Non Crystal.
From pediaa.com
What is the Difference Between Crystals and Gemstones Difference Between Crystal And Non Crystal By definition (with just a few special exceptions) minerals must be crystalline. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. This means that they are solids with an orderly repetitive atomic arrangement. Crystal, any. Difference Between Crystal And Non Crystal.
From www.youtube.com
Crystalline Solid and Non Crystalline Solid Crystalline Solid Vs Difference Between Crystal And Non Crystal Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. This means that they are solids with an orderly repetitive atomic arrangement. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. By definition (with just a few special exceptions) minerals. Difference Between Crystal And Non Crystal.
From howtofindrocks.com
Rock, Mineral, or Crystal? What’s the Difference? How to Find Rocks Difference Between Crystal And Non Crystal This means that they are solids with an orderly repetitive atomic arrangement. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. Crystalline structure has atoms arranged in order whereas. The major difference between crystalline and amorphous is crystalline solid is anisotropic. Difference Between Crystal And Non Crystal.
From askanydifference.com
Cristal vs Mineral Diferencia y Comparación Difference Between Crystal And Non Crystal The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. This means that they are solids with an orderly repetitive atomic arrangement. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. By definition (with just a few special exceptions) minerals must be crystalline. Crystalline solids and noncrystalline solids are. Difference Between Crystal And Non Crystal.
From askanydifference.com
Crystal vs Crystalline Difference and Comparison Difference Between Crystal And Non Crystal Crystalline structure has atoms arranged in order whereas. This means that they are solids with an orderly repetitive atomic arrangement. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. By. Difference Between Crystal And Non Crystal.
From www.hobbyistgeek.com
What Is The Difference Between Gemstone And Crystal? "Revealed" Difference Between Crystal And Non Crystal Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. By definition (with just a few special exceptions) minerals must be crystalline. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. This means that they are solids with an orderly repetitive atomic arrangement. Crystal, any. Difference Between Crystal And Non Crystal.
From www.pinterest.com
Crystal Identification Chart No. 3 Crystal identification, Minerals Difference Between Crystal And Non Crystal The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. By definition (with just a few special exceptions) minerals must be crystalline. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. Crystal, any. Difference Between Crystal And Non Crystal.
From www.toppr.com
Crystalline and Amorphous Solids Explanation, Differences, Examples, etc Difference Between Crystal And Non Crystal The basic difference between crystalline and noncrystalline structures is their atomic arrangement. This means that they are solids with an orderly repetitive atomic arrangement. By definition (with just a few special exceptions) minerals must be crystalline. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Crystalline. Difference Between Crystal And Non Crystal.
From askanydifference.com
Crystal vs Mineral Difference and Comparison Difference Between Crystal And Non Crystal Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. Crystalline structure has atoms arranged in order whereas. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. By definition (with just a few special exceptions) minerals must be crystalline. Crystal, any solid material in which. Difference Between Crystal And Non Crystal.
From pediaa.com
Difference Between Atomic Structure and Crystal Structure Definition Difference Between Crystal And Non Crystal The basic difference between crystalline and noncrystalline structures is their atomic arrangement. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. This means that they are solids with an orderly repetitive atomic arrangement. By definition. Difference Between Crystal And Non Crystal.
From www.differencebetween.com
Difference Between Minerals and Crystals Compare the Difference Difference Between Crystal And Non Crystal This means that they are solids with an orderly repetitive atomic arrangement. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. Crystalline structure has atoms arranged in order whereas. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. Crystalline solids and noncrystalline solids are two distinct types of. Difference Between Crystal And Non Crystal.
From firstyearengineer.com
Crystal Structure the first year engineer Difference Between Crystal And Non Crystal Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Crystalline structure has atoms arranged in order whereas. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is. Difference Between Crystal And Non Crystal.
From slideplayer.com
Chapter 3 Minerals. ppt download Difference Between Crystal And Non Crystal Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. By definition (with just a few special exceptions) minerals must be crystalline. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. Crystalline structure has atoms arranged in order whereas. This. Difference Between Crystal And Non Crystal.
From www.geologyin.com
Crystal Structure and Crystal Systems Difference Between Crystal And Non Crystal The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. Crystalline structure has atoms arranged in order whereas. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. By definition (with just a few special exceptions) minerals must be. Difference Between Crystal And Non Crystal.
From www.pinterest.ca
The difference between rocks, minerals, and gems. Minerals, Minerals Difference Between Crystal And Non Crystal By definition (with just a few special exceptions) minerals must be crystalline. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. Crystalline structure has atoms arranged in order whereas. This means that they are solids. Difference Between Crystal And Non Crystal.
From jaylenkruwkerr.blogspot.com
Explain Differences Between Single Crystal and Polycrystalline Material Difference Between Crystal And Non Crystal This means that they are solids with an orderly repetitive atomic arrangement. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. By definition (with just a few special exceptions) minerals must be crystalline. The major difference between crystalline and amorphous is crystalline solid is anisotropic where. Difference Between Crystal And Non Crystal.
From www.askdifference.com
Crystal vs. Cristal — What’s the Difference? Difference Between Crystal And Non Crystal By definition (with just a few special exceptions) minerals must be crystalline. This means that they are solids with an orderly repetitive atomic arrangement. Crystalline structure has atoms arranged in order whereas. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. Crystalline solids and noncrystalline solids are two distinct types of. Difference Between Crystal And Non Crystal.
From www.examplesof.net
Example of Solids Crystalline solids and Amorphous solids Difference Between Crystal And Non Crystal Crystalline structure has atoms arranged in order whereas. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. This means that they are solids with an orderly repetitive atomic arrangement. By definition (with just a few special exceptions) minerals must be crystalline. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid. Difference Between Crystal And Non Crystal.
From askanydifference.com
Crystal vs Gem Difference and Comparison Difference Between Crystal And Non Crystal Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. This means that they are solids with an orderly repetitive atomic arrangement. Crystalline solids and noncrystalline solids are two distinct types of solid materials with. Difference Between Crystal And Non Crystal.
From differencesfinder.com
Difference Between Crystals and Gems Differences Finder Difference Between Crystal And Non Crystal Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. The major difference between crystalline and amorphous is crystalline. Difference Between Crystal And Non Crystal.
From msestudent.com
What is the Difference Between “Crystal Structure” and “Bravais Lattice Difference Between Crystal And Non Crystal This means that they are solids with an orderly repetitive atomic arrangement. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Crystalline structure has atoms arranged in order whereas. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is. Difference Between Crystal And Non Crystal.
From eduinput.com
7 Difference between crystalline solids and amorphous solids Difference Between Crystal And Non Crystal Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. Crystalline structure has atoms arranged in order whereas. This means that they are solids with an orderly repetitive atomic arrangement. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. By. Difference Between Crystal And Non Crystal.
From www.allcrystal.com
Crystal vs. Glass What Are the Differences? All Crystal Difference Between Crystal And Non Crystal The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. Crystalline structure has atoms arranged in order whereas. This means that they are solids with an orderly repetitive atomic arrangement. By definition (with just a few special exceptions) minerals must be crystalline. Crystal, any solid material in which the component atoms are. Difference Between Crystal And Non Crystal.
From www.slideserve.com
PPT Lecture 9 Rocks and Minerals PowerPoint Presentation, free Difference Between Crystal And Non Crystal The basic difference between crystalline and noncrystalline structures is their atomic arrangement. By definition (with just a few special exceptions) minerals must be crystalline. Crystal, any solid material in which the component atoms are arranged in a definite pattern and whose surface regularity reflects its internal symmetry. Crystalline structure has atoms arranged in order whereas. This means that they are. Difference Between Crystal And Non Crystal.
From www.meritnation.com
Differencebetween unit cell and crystal lattice Chemistry The Solid Difference Between Crystal And Non Crystal Crystalline structure has atoms arranged in order whereas. Crystalline solids and noncrystalline solids are two distinct types of solid materials with different structural arrangements. The major difference between crystalline and amorphous is crystalline solid is anisotropic where as amorphous solid is isotropic. The basic difference between crystalline and noncrystalline structures is their atomic arrangement. This means that they are solids. Difference Between Crystal And Non Crystal.