Copper Iodide Solution Colour . Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states. Find out how to use copper (ii). Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. It is made by reacting iodine and copper in concentrated. You’re correct that this is just a suspension of copper(i) iodide. Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. If cui were soluble in water, the solution would be clear; Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. See examples of hexaaqua metal ions and how ligands affect the.
from slidetodoc.com
Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. It is made by reacting iodine and copper in concentrated. Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. See examples of hexaaqua metal ions and how ligands affect the. If cui were soluble in water, the solution would be clear; Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states. Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. Find out how to use copper (ii). Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. You’re correct that this is just a suspension of copper(i) iodide.
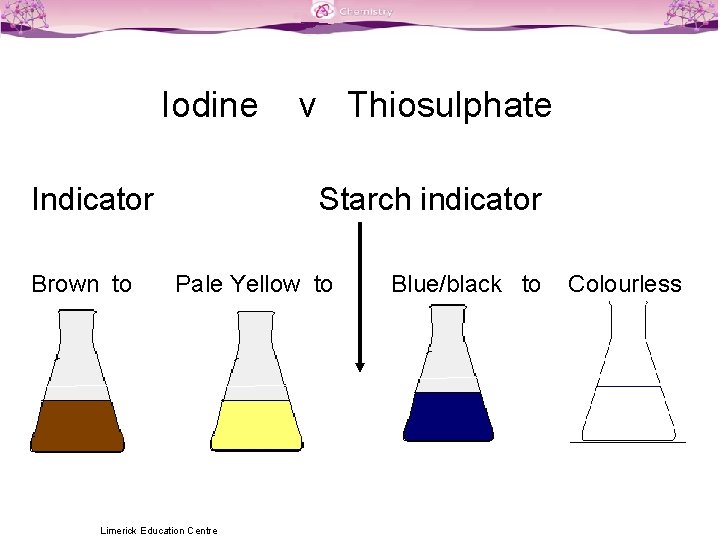
Titration Colour Changes SLSS Science Limerick Education Centre
Copper Iodide Solution Colour Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. See examples of hexaaqua metal ions and how ligands affect the. Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. It is made by reacting iodine and copper in concentrated. Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. You’re correct that this is just a suspension of copper(i) iodide. Find out how to use copper (ii). Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. If cui were soluble in water, the solution would be clear; Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states.
From www.nanochemazone.com
Copper(I) Iodide Powder Low Price 1 highly pure Nanochemazone Copper Iodide Solution Colour Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. You’re correct that this is just a suspension of copper(i) iodide. Learn about the reactions and properties of copper (ii) and copper (i) ions in. Copper Iodide Solution Colour.
From ar.inspiredpencil.com
Potassium Iodide Solution Color Copper Iodide Solution Colour If cui were soluble in water, the solution would be clear; You’re correct that this is just a suspension of copper(i) iodide. See examples of hexaaqua metal ions and how ligands affect the. Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. Learn about the properties, occurrence, extraction and reactions of. Copper Iodide Solution Colour.
From www.pinterest.com
Something really interesting Fluorescence thermochromism of copper(I)iodidepyridine complex Copper Iodide Solution Colour Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. It is made by reacting iodine and copper in concentrated. Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities.. Copper Iodide Solution Colour.
From www.indiamart.com
Copper Iodide Powder at Rs 700/kg Iodine Derivatives in Palghar ID 2850822013591 Copper Iodide Solution Colour You’re correct that this is just a suspension of copper(i) iodide. Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. See examples of hexaaqua metal ions and how. Copper Iodide Solution Colour.
From www.sciencephoto.com
Copper (I) iodide precipitate Stock Image A500/0573 Science Photo Library Copper Iodide Solution Colour If cui were soluble in water, the solution would be clear; Find out how to use copper (ii). Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states. See examples of hexaaqua. Copper Iodide Solution Colour.
From slidetodoc.com
Copper sulfate solution and potassium iodide solution Blue Copper Iodide Solution Colour See examples of hexaaqua metal ions and how ligands affect the. Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to. Copper Iodide Solution Colour.
From www.sciencephoto.com
Copper ion solutions Stock Image A500/0402 Science Photo Library Copper Iodide Solution Colour Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. See examples of hexaaqua metal ions and how ligands affect the. You’re correct that this is just a suspension of copper(i) iodide. Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high. Copper Iodide Solution Colour.
From www.youtube.com
Empirical Formula of Copper Iodide Virtual Lab YouTube Copper Iodide Solution Colour If cui were soluble in water, the solution would be clear; Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states. See examples of hexaaqua metal ions. Copper Iodide Solution Colour.
From slidetodoc.com
Copper sulfate solution and potassium iodide solution Blue Copper Iodide Solution Colour Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states. Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. It is made by reacting iodine and copper in concentrated. If cui were soluble in water, the solution would be clear; Copper(i). Copper Iodide Solution Colour.
From www.researchgate.net
Hand specimen of the copper iodide and iodatebearing (redcolored... Download Scientific Diagram Copper Iodide Solution Colour Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. It is made by reacting iodine and copper in concentrated. You’re correct that this is just a suspension of copper(i) iodide. See examples of hexaaqua metal ions and how ligands affect the. Copper(i) iodide is white, but samples are often tan or. Copper Iodide Solution Colour.
From ar.inspiredpencil.com
Potassium Iodide Solution Color Copper Iodide Solution Colour Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. It is made by reacting iodine and copper in concentrated. See examples of hexaaqua metal ions and how ligands affect the. Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. If cui were soluble. Copper Iodide Solution Colour.
From klaexmnbf.blob.core.windows.net
Copper Iodide Redox Reaction at Robert Marx blog Copper Iodide Solution Colour See examples of hexaaqua metal ions and how ligands affect the. Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. Learn about the properties,. Copper Iodide Solution Colour.
From www.fishersci.com
Cuprous Iodide, 98, Spectrum Fisher Scientific Copper Iodide Solution Colour Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in. Copper Iodide Solution Colour.
From www.ttgnet.com
Week of 30 July 2007 Copper Iodide Solution Colour Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. You’re correct that this is just a suspension of copper(i) iodide. It is made by. Copper Iodide Solution Colour.
From thehomescientist.blogspot.com
The Home Scientist The Long Road to Copper (I) Iodide Copper Iodide Solution Colour Find out how to use copper (ii). If cui were soluble in water, the solution would be clear; Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states. Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. You’re correct that this is. Copper Iodide Solution Colour.
From thehomescientist.blogspot.com
The Home Scientist The Long Road to Copper (I) Iodide Copper Iodide Solution Colour Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. See examples of hexaaqua metal ions and how ligands affect the. Find out how to use copper (ii). You’re correct that this is just a suspension of copper(i) iodide. Learn how copper cations (cu+ and cu2+) and. Copper Iodide Solution Colour.
From pubs.acs.org
Cesium Copper Iodide Tailored Nanoplates and Nanorods for Blue, Yellow, and White Emission Copper Iodide Solution Colour If cui were soluble in water, the solution would be clear; It is made by reacting iodine and copper in concentrated. Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. Learn how copper cations (cu+ and cu2+) and their compounds and complexes. Copper Iodide Solution Colour.
From www.numerade.com
SOLVED Calculate the solubility of the CuI (copper iodide) solid in 0.100 M KI solution in Copper Iodide Solution Colour Find out how to use copper (ii). Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states. Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. Copper(i) iodide is white, but samples are often tan or. Copper Iodide Solution Colour.
From www.nanochemazone.com
Copper(I) Iodide Powder Low Price 1 highly pure Nanochemazone Copper Iodide Solution Colour See examples of hexaaqua metal ions and how ligands affect the. Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. Find out how to use copper (ii). Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due. Copper Iodide Solution Colour.
From thehomescientist.blogspot.com
The Home Scientist The Long Road to Copper (I) Iodide Copper Iodide Solution Colour It is made by reacting iodine and copper in concentrated. Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states. Find out how. Copper Iodide Solution Colour.
From chemistry.com.pk
The Chemistry Of Colored Glass Copper Iodide Solution Colour You’re correct that this is just a suspension of copper(i) iodide. If cui were soluble in water, the solution would be clear; Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states. See examples of hexaaqua metal ions and how ligands affect the. Learn about the reactions and properties of. Copper Iodide Solution Colour.
From www.sciencephoto.com
Copper (I) iodide precipitate Stock Image C030/7214 Science Photo Library Copper Iodide Solution Colour Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states. It is made by reacting iodine and copper in concentrated. Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. If cui were soluble in water, the. Copper Iodide Solution Colour.
From www.labkafe.com
Color of Common Salts Used in School Laboratories Labkafe Copper Iodide Solution Colour See examples of hexaaqua metal ions and how ligands affect the. Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and. Copper Iodide Solution Colour.
From thehomescientist.blogspot.com
The Home Scientist The Long Road to Copper (I) Iodide Copper Iodide Solution Colour See examples of hexaaqua metal ions and how ligands affect the. Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. If cui were soluble in water, the solution would be clear; Learn about the. Copper Iodide Solution Colour.
From www.youtube.com
Iodometric Titration of Copper YouTube Copper Iodide Solution Colour Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. See examples of hexaaqua metal ions and how ligands affect the. Find out how to use copper (ii). It is made by reacting iodine and copper in concentrated. Learn about the properties, occurrence,. Copper Iodide Solution Colour.
From www.sagarlifescience.com
Copper Iodide SagarLifeScience Copper Iodide Solution Colour Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states. Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. You’re correct that this is just a suspension of copper(i) iodide. Copper (i) iodide is a white. Copper Iodide Solution Colour.
From www.youtube.com
How to Write the Formula for Copper (I) iodide YouTube Copper Iodide Solution Colour Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. Find out how to use copper (ii). Copper(i) iodide is white, but samples are often tan or even, when found. Copper Iodide Solution Colour.
From valerianlabs.com
Copper (I) Iodide Valerian Labs Chemical Store Copper Iodide Solution Colour You’re correct that this is just a suspension of copper(i) iodide. Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states. Copper (i). Copper Iodide Solution Colour.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Copper Iodide Solution Colour If cui were soluble in water, the solution would be clear; Find out how to use copper (ii). It is made by reacting iodine and copper in concentrated. Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. Learn about the reactions and properties of copper (ii) and copper (i) ions in solution,. Copper Iodide Solution Colour.
From www.slideserve.com
PPT Chem 3024 Fall 2003 Iodometric Determination of Copper PowerPoint Presentation ID149780 Copper Iodide Solution Colour Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in. Copper Iodide Solution Colour.
From www.youtube.com
Iodine and Copper II Chloride YouTube Copper Iodide Solution Colour It is made by reacting iodine and copper in concentrated. Find out how to use copper (ii). Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in. Copper Iodide Solution Colour.
From www.youtube.com
Empirical Formula of Copper Iodide Virtual Lab Student B YouTube Copper Iodide Solution Colour Learn how copper cations (cu+ and cu2+) and their compounds and complexes have different colours in solid and aqueous states. Learn about the reactions and properties of copper (ii) and copper (i) ions in solution, including colour changes, precipitation, ligand exchange and disproportionation. You’re correct that this is just a suspension of copper(i) iodide. Copper (i) iodide is a white. Copper Iodide Solution Colour.
From www.sciencephoto.com
Copper (I) iodide precipitate Stock Image C030/7215 Science Photo Library Copper Iodide Solution Colour Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. See examples of hexaaqua metal ions and how ligands affect the. Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. Find out how to. Copper Iodide Solution Colour.
From ar.inspiredpencil.com
Potassium Iodide Solution Color Copper Iodide Solution Colour Copper(i) iodide is white, but samples are often tan or even, when found in nature as mineral marshite, reddish brown, but such color is due to impurities. It is made by reacting iodine and copper in concentrated. Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. Learn how copper cations (cu+ and. Copper Iodide Solution Colour.
From www.chegg.com
Name of the Experiment Estimation of copper in the Copper Iodide Solution Colour Copper (i) iodide is a white solid that is a reducing agent and easily turns tan or brown. Find out how to use copper (ii). Learn about the properties, occurrence, extraction and reactions of copper, a transition metal with high conductivity and. You’re correct that this is just a suspension of copper(i) iodide. Learn about the reactions and properties of. Copper Iodide Solution Colour.