Mhra Medical Devices Ce Marking . Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device labeling for products sold. Ukca marking deadline for medical devices & ivds. Currently, ce marked medical devices with a valid ce mark can be placed on the great britain (gb) market until 30 june 2023. Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. We will continue to work with industry,. Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment).
from www.ce-marking.com
Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. Currently, ce marked medical devices with a valid ce mark can be placed on the great britain (gb) market until 30 june 2023. Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device labeling for products sold. Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. We will continue to work with industry,. Ukca marking deadline for medical devices & ivds. This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment). Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain.
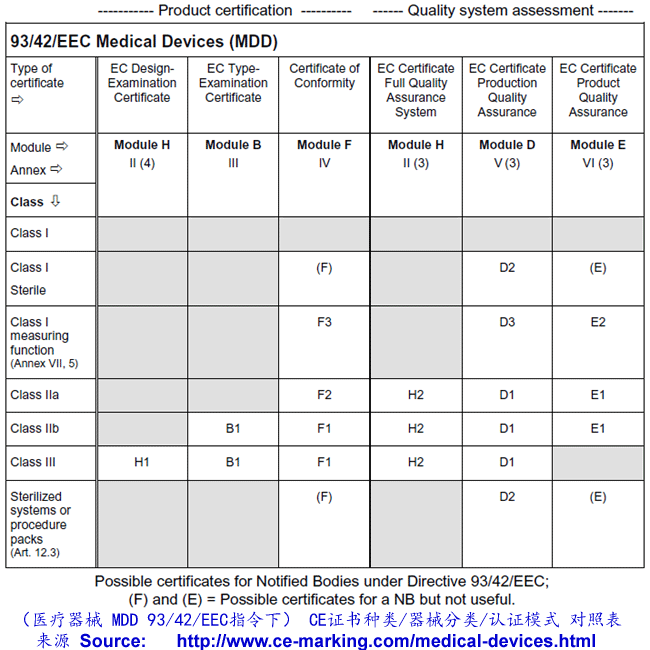
Guide on Class III MDD Medical Devices CE marking (mark) & European
Mhra Medical Devices Ce Marking We will continue to work with industry,. Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. Ukca marking deadline for medical devices & ivds. We will continue to work with industry,. Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment). Currently, ce marked medical devices with a valid ce mark can be placed on the great britain (gb) market until 30 june 2023. Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device labeling for products sold.
From us.operonstrategist.com
CE Mark for Medical Devices (StepbyStep Process) Operon Strategist Mhra Medical Devices Ce Marking Ukca marking deadline for medical devices & ivds. This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment). Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. Certificates that have been extended will also. Mhra Medical Devices Ce Marking.
From www.youtube.com
CE Marking Process as per EU MDR (European Medical Device Regulation Mhra Medical Devices Ce Marking Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain. Mhra Medical Devices Ce Marking.
From www.ivdeology.co.uk
Clarity for CE marking on IVD's and Medical Devices, Specialist Quality Mhra Medical Devices Ce Marking Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. Currently, ce marked medical devices with a valid ce mark can be placed on the great britain (gb) market until 30 june 2023. We will continue to work with industry,. Ukca marking deadline for medical devices & ivds. Mhra guidance. Mhra Medical Devices Ce Marking.
From us.operonstrategist.com
CE Mark for Medical Devices (StepbyStep Process) Operon Strategist Mhra Medical Devices Ce Marking This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment). Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. Currently, ce marked medical devices with a valid ce mark can be placed. Mhra Medical Devices Ce Marking.
From www.elexes.com
CE Marking Strategy For Medical Devices (EU CE Mark) Mhra Medical Devices Ce Marking Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device labeling for products sold.. Mhra Medical Devices Ce Marking.
From www.youtube.com
How to get your CE Mark Certification for Medical Devices ? YouTube Mhra Medical Devices Ce Marking Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. Ukca marking deadline for medical devices & ivds. Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device labeling for products sold. Mhra guidance has been updated to reflect these planned changes to the acceptance. Mhra Medical Devices Ce Marking.
From simplerqms.com
CE Marking for Medical Devices [StepbyStep Guide] Mhra Medical Devices Ce Marking Ukca marking deadline for medical devices & ivds. Currently, ce marked medical devices with a valid ce mark can be placed on the great britain (gb) market until 30 june 2023. We will continue to work with industry,. Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device labeling for products sold. Mhra guidance has. Mhra Medical Devices Ce Marking.
From sterlingmedicaldevices.com
CE Marking for Medical Devices Mhra Medical Devices Ce Marking Ukca marking deadline for medical devices & ivds. Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device labeling for products sold. Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. Currently, ce marked medical devices with a valid ce mark can be placed. Mhra Medical Devices Ce Marking.
From knobbemedical.com
MHRA Updates Guidance on Healthcare Apps as Medical Devices Knobbe Mhra Medical Devices Ce Marking Currently, ce marked medical devices with a valid ce mark can be placed on the great britain (gb) market until 30 june 2023. Ukca marking deadline for medical devices & ivds. Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. Medical device manufacturers must apply a new uk conformity assessed. Mhra Medical Devices Ce Marking.
From www.i3cglobal.com
UKCA Marking Medical Devices MHRA Registration I3C Mhra Medical Devices Ce Marking Currently, ce marked medical devices with a valid ce mark can be placed on the great britain (gb) market until 30 june 2023. We will continue to work with industry,. Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. This mhra link provides a graphic with the timelines for placement. Mhra Medical Devices Ce Marking.
From www.compliancequest.com
A Guide to CE Marking for Medical Devices Mhra Medical Devices Ce Marking Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. Ukca marking deadline for medical devices & ivds. Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. Certificates that have been extended will also be recognised as valid for. Mhra Medical Devices Ce Marking.
From ce-marking.com
Guide on AIMDActive Implantable Medical Devices CE marking (mark Mhra Medical Devices Ce Marking This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment). Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their. Mhra Medical Devices Ce Marking.
From www.i3cglobal.com
CE Marking Approval For Medical Device I3CGlobal Mhra Medical Devices Ce Marking Ukca marking deadline for medical devices & ivds. Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. We will continue to work with industry,. Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. Currently, ce marked medical. Mhra Medical Devices Ce Marking.
From medicaldevicehq.com
CEmark a medical device How do you do it? Mhra Medical Devices Ce Marking Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device labeling for products sold. Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. Certificates. Mhra Medical Devices Ce Marking.
From www.linkedin.com
UKCA Marking & the MHRA Mhra Medical Devices Ce Marking Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment). Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their. Mhra Medical Devices Ce Marking.
From www.ce-marking.com
Guide on Class III MDD Medical Devices CE marking (mark) & European Mhra Medical Devices Ce Marking Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. Ukca marking deadline for medical devices & ivds. Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device labeling for products sold. Mhra guidance has been updated to reflect these planned changes to the acceptance. Mhra Medical Devices Ce Marking.
From casusconsulting.com
Aug2023 UK MHRA Clarifies CE Marking Timeline Casus Consulting Mhra Medical Devices Ce Marking Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device labeling for products sold. Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. We. Mhra Medical Devices Ce Marking.
From www.sgs-cqe.de
CE Marking of Medical Devices Mhra Medical Devices Ce Marking Ukca marking deadline for medical devices & ivds. Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment). Mhra guidance has been updated to. Mhra Medical Devices Ce Marking.
From www.formly.ai
Navigating CE Marking for Medical Devices A CostEffective Roadmap for Mhra Medical Devices Ce Marking Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment). Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device. Mhra Medical Devices Ce Marking.
From fdapals.com
Medical Device CE Marking FDApals Mhra Medical Devices Ce Marking Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment). Currently, ce marked medical devices with a valid ce mark can be placed on. Mhra Medical Devices Ce Marking.
From www.ce-marking.eu
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark Mhra Medical Devices Ce Marking Currently, ce marked medical devices with a valid ce mark can be placed on the great britain (gb) market until 30 june 2023. Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device labeling for products sold. Ukca marking deadline for medical devices & ivds. This mhra link provides a graphic with the timelines for. Mhra Medical Devices Ce Marking.
From www.ce-marking.eu
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark Mhra Medical Devices Ce Marking We will continue to work with industry,. Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. Currently, ce marked medical devices with a valid ce mark can be placed on the great britain (gb) market until 30 june 2023. Ukca marking deadline for medical devices & ivds. Medical device. Mhra Medical Devices Ce Marking.
From www.youtube.com
Process to Get CE Certification for Medical Devices CE Marking Mhra Medical Devices Ce Marking Ukca marking deadline for medical devices & ivds. Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. Currently, ce marked medical devices with a valid ce mark can. Mhra Medical Devices Ce Marking.
From blog.sierralabs.com
5 Steps to Obtain a CE Marking on Your Medical Device Mhra Medical Devices Ce Marking Currently, ce marked medical devices with a valid ce mark can be placed on the great britain (gb) market until 30 june 2023. This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment). Independent regulation of ce marking of medical devices on 1 august. Mhra Medical Devices Ce Marking.
From www.ce-marking.com
CE mark medical device medical devices CE marking Mhra Medical Devices Ce Marking This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment). Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. We will continue to work with industry,. Medical device manufacturers must apply a. Mhra Medical Devices Ce Marking.
From operonstrategist.com
A Comprehensive Guide to MHRA Medical Device Registration (Steps Mhra Medical Devices Ce Marking Currently, ce marked medical devices with a valid ce mark can be placed on the great britain (gb) market until 30 june 2023. Ukca marking deadline for medical devices & ivds. Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. Certificates that have been extended will also be. Mhra Medical Devices Ce Marking.
From www.aplyon.com
CE Marking Procedure Mhra Medical Devices Ce Marking Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. Ukca marking deadline for medical devices & ivds. We will continue to work with industry,. This mhra link provides. Mhra Medical Devices Ce Marking.
From www.medicalplasticsnews.com
How to get a CE Mark from the MHRA Medical Plastics News Mhra Medical Devices Ce Marking Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. We will continue to work with industry,. Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. This mhra link provides a graphic with the timelines for placement of ce marked. Mhra Medical Devices Ce Marking.
From www.lexology.com
The MHRA's recent updates to the regulation of medical devices Lexology Mhra Medical Devices Ce Marking Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. We will continue to work with industry,. Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. This mhra link provides a graphic with the timelines for placement of ce. Mhra Medical Devices Ce Marking.
From www.youtube.com
7 Steps How to Get a CE Marking Certification for Medical Devices Mhra Medical Devices Ce Marking Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. Currently, ce marked medical devices with a valid ce mark can be placed on the great britain (gb) market until 30 june 2023. Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published. Mhra Medical Devices Ce Marking.
From mavenprofserv.us
Demystifying Medical Device CE Marking for Peak Performance Mhra Medical Devices Ce Marking Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. We will continue to work with industry,. Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device labeling for products sold. Currently, ce marked medical devices with a valid ce mark can be placed on the. Mhra Medical Devices Ce Marking.
From www.compliancequest.com
A Guide to CE Marking for Medical Devices Mhra Medical Devices Ce Marking Certificates that have been extended will also be recognised as valid for placing ce marked devices on the gb market. Mhra guidance has been updated to reflect these planned changes to the acceptance of ce marked medical devices in great britain. Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on.. Mhra Medical Devices Ce Marking.
From operonstrategist.com
European CE Mark for Medical Devices CE Certification Guidance (Quick Mhra Medical Devices Ce Marking Medical device manufacturers must apply a new uk conformity assessed (ukca) mark to their device labeling for products sold. We will continue to work with industry,. Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. Ukca marking deadline for medical devices & ivds. Mhra guidance has been updated to reflect. Mhra Medical Devices Ce Marking.
From www.fiverr.com
Provide mhra, ce marking, ukca, eudamed, mdr and doc by Komal_chaudhry Mhra Medical Devices Ce Marking This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment). Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. Ukca marking deadline for medical devices & ivds. Currently, ce marked medical devices with a. Mhra Medical Devices Ce Marking.
From operonstrategist.com
A Comprehensive Guide to MHRA Medical Device Registration (Steps Mhra Medical Devices Ce Marking We will continue to work with industry,. Independent regulation of ce marking of medical devices on 1 august 2023, the uk government published a statement on. This mhra link provides a graphic with the timelines for placement of ce marked medical devices on the great britain market under the medical devices (amendment). Medical device manufacturers must apply a new uk. Mhra Medical Devices Ce Marking.