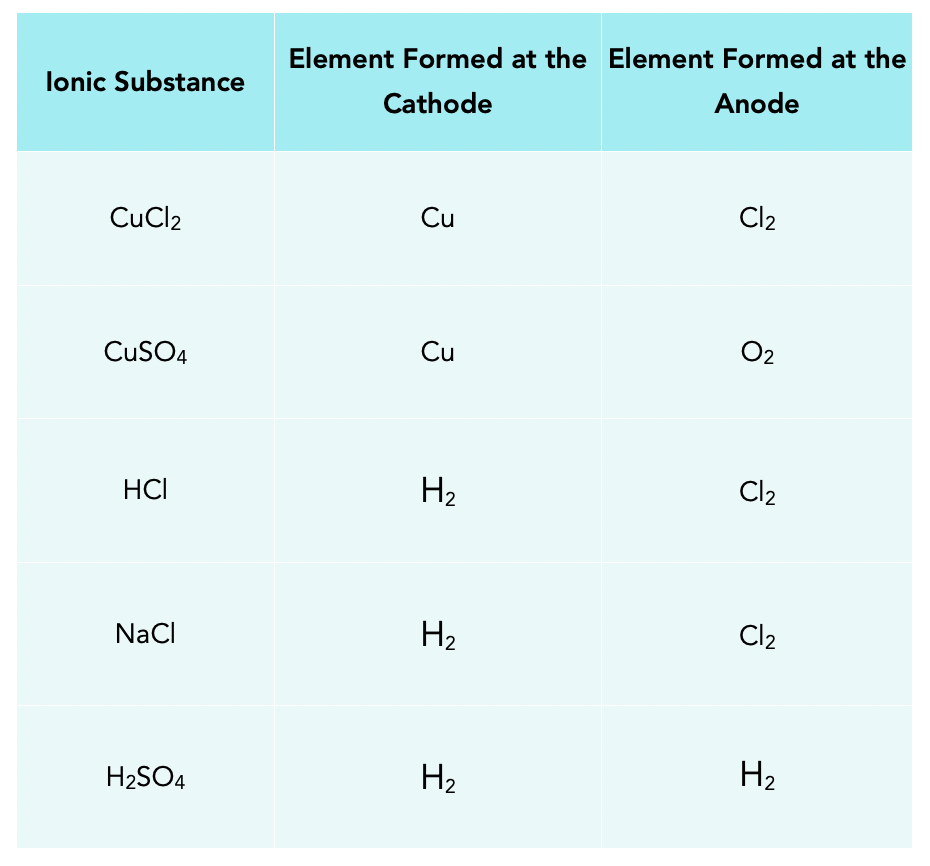
Table Salt Electrolysis Chlorine . Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. I was looking up methods to create hcl and came across this video: The electrolytic cell used in the process is. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. This seems like a simple method but i was wondering about the. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by.
from studymind.co.uk
I was looking up methods to create hcl and came across this video: Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. The electrolytic cell used in the process is. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. This seems like a simple method but i was wondering about the. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas.
Electrolysis of Aqueous Solutions (GCSE Chemistry) Study Mind
Table Salt Electrolysis Chlorine This seems like a simple method but i was wondering about the. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. The electrolytic cell used in the process is. I was looking up methods to create hcl and came across this video: This seems like a simple method but i was wondering about the. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although.
From www.gradegorilla.com
Gradegorilla Chemistry Table Salt Electrolysis Chlorine This seems like a simple method but i was wondering about the. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is. Table Salt Electrolysis Chlorine.
From general.chemistrysteps.com
Electrolysis Chemistry Steps Table Salt Electrolysis Chlorine I was looking up methods to create hcl and came across this video: Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. For reference, electrochlorination is just the electrolysis of a simple solution of water. Table Salt Electrolysis Chlorine.
From www.youtube.com
IC15 Electrolysis of sodium chloride solution YouTube Table Salt Electrolysis Chlorine The electrolytic cell used in the process is. I was looking up methods to create hcl and came across this video: Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although.. Table Salt Electrolysis Chlorine.
From elchoroukhost.net
Chemical Equation For Table Salt And Water Elcho Table Table Salt Electrolysis Chlorine Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. I was looking up methods to create hcl and came across this video: This seems like a simple method but i was wondering about the. Electrolysis of. Table Salt Electrolysis Chlorine.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Table Salt Electrolysis Chlorine Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. I was looking up methods to create hcl and came across this video: The electrolytic cell used in the process is. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Molten. Table Salt Electrolysis Chlorine.
From chemrevisionigcse.blogspot.com
IGCSE Chemistry Electrolysis Table Salt Electrolysis Chlorine For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. I was looking up methods to create. Table Salt Electrolysis Chlorine.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Table Salt Electrolysis Chlorine For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. The electrolytic cell. Table Salt Electrolysis Chlorine.
From mavink.com
Electrolysis Of Aqueous Sodium Chloride Table Salt Electrolysis Chlorine For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. I was looking up methods to create hcl and came across this video: This seems like a simple method but i was wondering about the. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known. Table Salt Electrolysis Chlorine.
From www.onlinemathlearning.com
Chemical Reactions IGCSE Chemistry (solutions, examples, worksheets Table Salt Electrolysis Chlorine Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. I was looking up methods to create. Table Salt Electrolysis Chlorine.
From www.youtube.com
Electrolysis of Salt Solution at Home in Under 1 minute to produce Table Salt Electrolysis Chlorine Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. This seems like a simple method but i was wondering about the. I was looking up methods to create hcl and came across this video: Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. For reference,. Table Salt Electrolysis Chlorine.
From www.tessshebaylo.com
Salt Water Electrolysis Equation Tessshebaylo Table Salt Electrolysis Chlorine This seems like a simple method but i was wondering about the. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. For reference, electrochlorination is just the electrolysis of a. Table Salt Electrolysis Chlorine.
From mavink.com
Sodium Chloride Electrolysis Equation Table Salt Electrolysis Chlorine This seems like a simple method but i was wondering about the. The electrolytic cell used in the process is. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. For. Table Salt Electrolysis Chlorine.
From ar.inspiredpencil.com
Chlorine Diagram Table Salt Electrolysis Chlorine For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. The electrolytic cell used in the process is. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces. Table Salt Electrolysis Chlorine.
From ijmmm.ustb.edu.cn
Applications of molten salt and progress of molten salt electrolysis in Table Salt Electrolysis Chlorine For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. I was looking up methods to create hcl and came across this video: Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous. Table Salt Electrolysis Chlorine.
From courses.lumenlearning.com
Electrolysis Chemistry Table Salt Electrolysis Chlorine Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. The electrolytic cell used in the process is. This seems like a simple method but i was wondering about the. I was looking up methods to create. Table Salt Electrolysis Chlorine.
From www.alamy.com
Electrolysis of sodium chloride solution. Apparatus used to pass a Table Salt Electrolysis Chlorine I was looking up methods to create hcl and came across this video: Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. Electrolysis of an aqueous solution of table salt. Table Salt Electrolysis Chlorine.
From www.slideserve.com
PPT The Chemistry of Sodium Chloride PowerPoint Presentation ID578483 Table Salt Electrolysis Chlorine The electrolytic cell used in the process is. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. This seems like a simple method but i was wondering about the. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. I was looking up methods to create. Table Salt Electrolysis Chlorine.
From www.youtube.com
Electrolysis of Molten Sodium Chloride Half Reactions Electrolysis Table Salt Electrolysis Chlorine Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. I was looking up methods to create hcl. Table Salt Electrolysis Chlorine.
From www.dreamstime.com
The Electrolysis of Sodium Chloride. Vector Illustration Stock Vector Table Salt Electrolysis Chlorine Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. I was. Table Salt Electrolysis Chlorine.
From studymind.co.uk
Electrolysis of Aqueous Solutions (GCSE Chemistry) Study Mind Table Salt Electrolysis Chlorine The electrolytic cell used in the process is. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by.. Table Salt Electrolysis Chlorine.
From mungfali.com
Electrolysis Of Sodium Chloride Diagram Table Salt Electrolysis Chlorine For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. The electrolytic. Table Salt Electrolysis Chlorine.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Table Salt Electrolysis Chlorine Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. The electrolytic cell used in the process is. This seems like a simple method but i was wondering about the. For reference, electrochlorination is just the. Table Salt Electrolysis Chlorine.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Table Salt Electrolysis Chlorine Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. I was looking up methods to create hcl and came across this video: The electrolytic cell used in the process is. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Chlorine can be manufactured by the. Table Salt Electrolysis Chlorine.
From www.vecteezy.com
Electrolysis of Sodium Chloride solution 18891988 Vector Art at Vecteezy Table Salt Electrolysis Chlorine This seems like a simple method but i was wondering about the. I was looking up methods to create hcl and came across this video: Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. For. Table Salt Electrolysis Chlorine.
From courses.lumenlearning.com
Electrolysis Chemistry for Majors Table Salt Electrolysis Chlorine The electrolytic cell used in the process is. For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. I was looking up methods to create hcl and came across this video: Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process.. Table Salt Electrolysis Chlorine.
From www.researchgate.net
Electrochemical deoxidation of Ti in MgCl2HoCl3 molten salt Table Salt Electrolysis Chlorine The electrolytic cell used in the process is. For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali. Table Salt Electrolysis Chlorine.
From study.com
Electrolysis of Aqueous Solutions Lesson Table Salt Electrolysis Chlorine Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. The electrolytic cell used in the process is. I was looking up methods to create hcl and came across this video: This seems like a simple method. Table Salt Electrolysis Chlorine.
From mavink.com
Molten Sodium Chloride Electrolysis Table Salt Electrolysis Chlorine Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. The electrolytic cell used in the process is. I was looking up methods to create hcl and came across this video:. Table Salt Electrolysis Chlorine.
From mungfali.com
Electrolysis Of Sodium Chloride Diagram Table Salt Electrolysis Chlorine Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. This seems like a simple method but i. Table Salt Electrolysis Chlorine.
From www.essentialchemicalindustry.org
Chlorine Table Salt Electrolysis Chlorine The electrolytic cell used in the process is. For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although.. Table Salt Electrolysis Chlorine.
From revisechemistry.uk
Electrolysis AQA C4 revisechemistry.uk Table Salt Electrolysis Chlorine I was looking up methods to create hcl and came across this video: For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. This seems like a simple method but i was wondering about the. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known. Table Salt Electrolysis Chlorine.
From mavink.com
Electrolysis Of Sodium Chloride Table Salt Electrolysis Chlorine I was looking up methods to create hcl and came across this video: For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. This seems like a simple method but i was wondering about the. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide. Table Salt Electrolysis Chlorine.
From spj.science.org
CommonIon Effect Triggered Highly Sustained Seawater Electrolysis with Table Salt Electrolysis Chlorine The electrolytic cell used in the process is. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. I was looking up methods to create hcl and came across this video: Electrolysis of an aqueous solution of. Table Salt Electrolysis Chlorine.
From chemistrymsq10.blogspot.com
Grade10 CHAPTER 3 ELECTROLYSIS SEMESTER 1 Table Salt Electrolysis Chlorine For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. Chlorine can be manufactured by the electrolysis of a sodium chloride solution , which is known as the chloralkali process. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Molten (liquid). Table Salt Electrolysis Chlorine.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID9353258 Table Salt Electrolysis Chlorine The electrolytic cell used in the process is. For reference, electrochlorination is just the electrolysis of a simple solution of water and table salt (nacl) followed by. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous sodium hydroxide and chlorine, although. Electrolysis of an aqueous solution of table salt (nacl, or sodium chloride) produces aqueous. Table Salt Electrolysis Chlorine.