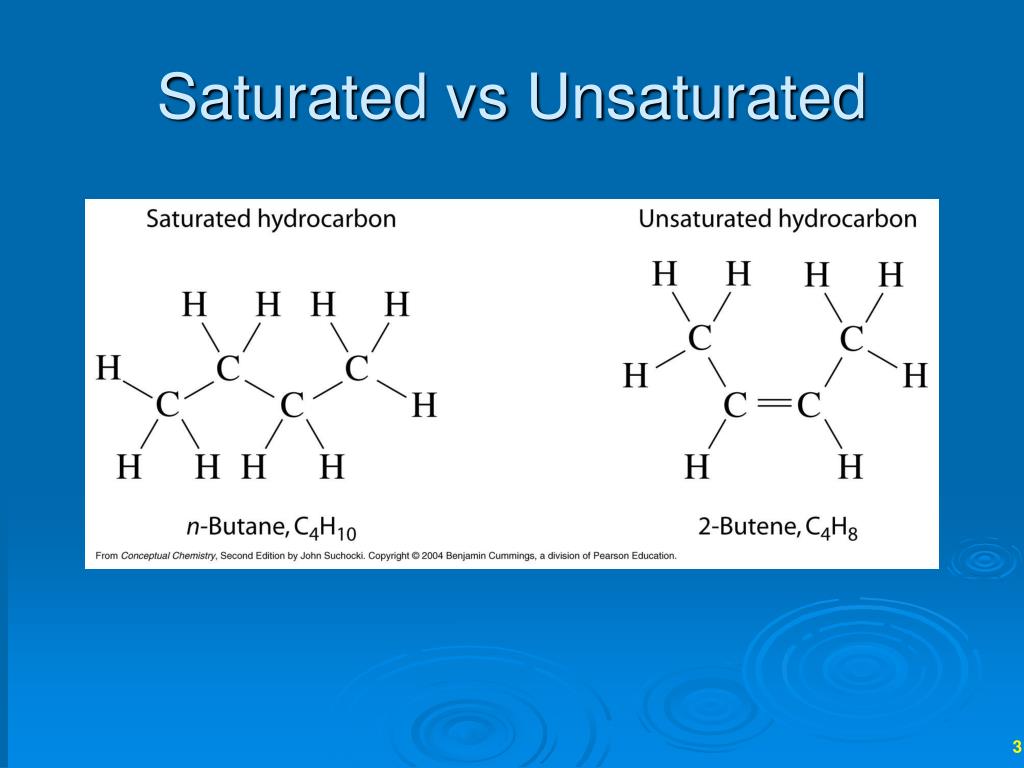
Unsaturated Examples Chemistry . examples of unsaturated solutions. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. an unsaturated solution is more dilute than a saturated solution. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. saturated and unsaturated compounds. unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. A saturated compound is a chemical compound (or ion) that resists addition. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. Examples of unsaturated organic molecules include hc=ch and h 2 c=o.
from www.slideserve.com
Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. examples of unsaturated solutions. saturated and unsaturated compounds. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. an unsaturated solution is more dilute than a saturated solution. A saturated compound is a chemical compound (or ion) that resists addition. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved.
PPT Organic Chemistry PowerPoint Presentation, free download ID5525521
Unsaturated Examples Chemistry A saturated compound is a chemical compound (or ion) that resists addition. unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. A saturated compound is a chemical compound (or ion) that resists addition. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. examples of unsaturated solutions. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. an unsaturated solution is more dilute than a saturated solution. saturated and unsaturated compounds. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution.
From www.youtube.com
Saturated Organic Compounds Vs Unsaturated Organic Compounds YouTube Unsaturated Examples Chemistry Examples of unsaturated organic molecules include hc=ch and h 2 c=o. an unsaturated solution is more dilute than a saturated solution. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. any solution with a concentration of solute below the saturation point of the solute is an unsaturated.. Unsaturated Examples Chemistry.
From guidelibperplexing.z13.web.core.windows.net
Diagram Of Unsaturated Fatty Acid Unsaturated Examples Chemistry unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. saturated and unsaturated compounds. an unsaturated solution is more dilute than a saturated solution. examples of unsaturated solutions. Apply a solubility conversion factor to calculate the amount of solute that can be. Unsaturated Examples Chemistry.
From www.slideshare.net
what is saturated,unsaturated and supersaturated solution Unsaturated Examples Chemistry unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. examples of unsaturated solutions. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. saturated and unsaturated compounds. any solution with a concentration of solute below the saturation point of the. Unsaturated Examples Chemistry.
From slidesharetrick.blogspot.com
What Is An Unsaturated Solution Apex slidesharetrick Unsaturated Examples Chemistry Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. saturated and unsaturated compounds. examples of unsaturated solutions. any solution with a. Unsaturated Examples Chemistry.
From jphechsi.blogspot.com
organic chemistry α,βunsaturated carbonyl compounds and alkyl addition Unsaturated Examples Chemistry Examples of unsaturated organic molecules include hc=ch and h 2 c=o. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. A saturated compound is a chemical compound (or ion) that resists. Unsaturated Examples Chemistry.
From www.teachoo.com
[Class 10] What are saturated & unsaturated hydrocarbon with examples? Unsaturated Examples Chemistry unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. examples of unsaturated solutions. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. A saturated compound is a chemical compound (or ion) that resists addition. Apply a solubility conversion factor to calculate. Unsaturated Examples Chemistry.
From www.ck12.org
Saturated, Unsaturated, Supersaturated Example 2 ( Video ) Chemistry CK12 Foundation Unsaturated Examples Chemistry saturated and unsaturated compounds. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. examples of unsaturated solutions. A saturated compound. Unsaturated Examples Chemistry.
From pediaa.com
Difference Between Saturated and Unsaturated Compounds Definition, Explanation, Examples and Unsaturated Examples Chemistry examples of unsaturated solutions. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation. Unsaturated Examples Chemistry.
From www.youtube.com
Saturated, Unsaturated, Supersaturated YouTube Unsaturated Examples Chemistry any solution with a concentration of solute below the saturation point of the solute is an unsaturated. an unsaturated solution is more dilute than a saturated solution. unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. Examples of unsaturated organic molecules include. Unsaturated Examples Chemistry.
From www.ruled.me
Unsaturated Fat What it is & Examples Guide] Unsaturated Examples Chemistry Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. A saturated compound is a chemical compound (or ion) that resists addition. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. saturated and. Unsaturated Examples Chemistry.
From www.youtube.com
Saturated Hydrocarbons and Unsaturated Hydrocarbons Class 10 Chemistry Chapter Organic Unsaturated Examples Chemistry Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. A saturated compound is a chemical compound (or ion) that resists addition. Examples of unsaturated organic molecules include hc=ch and h 2. Unsaturated Examples Chemistry.
From www.youtube.com
Woodward Fieser Rules for α, β Unsaturated Carbonyl compounds GA Chemistry YouTube Unsaturated Examples Chemistry Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. examples of unsaturated solutions. an unsaturated solution is a solution that contains less than the maximum amount of solute that. Unsaturated Examples Chemistry.
From www.youtube.com
What is the Difference between saturated hydrocarbons and unsaturated hydrocarbons Chemistry Unsaturated Examples Chemistry Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. saturated and unsaturated compounds. an unsaturated solution is more dilute than a saturated solution. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. unsaturated. Unsaturated Examples Chemistry.
From sciencenotes.org
Unsaturated Solution Definition and Examples in Chemistry Unsaturated Examples Chemistry A saturated compound is a chemical compound (or ion) that resists addition. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. an unsaturated solution is a solution that contains less than the maximum amount of solute that. Unsaturated Examples Chemistry.
From chem.libretexts.org
3.2 Nomenclature of unsaturated hydrocarbons Chemistry LibreTexts Unsaturated Examples Chemistry A saturated compound is a chemical compound (or ion) that resists addition. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. Adding a spoonful of sugar to. Unsaturated Examples Chemistry.
From www.vrogue.co
Unsaturated Hydrocarbon Alkenes Classnotes Ng vrogue.co Unsaturated Examples Chemistry Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. saturated and unsaturated compounds. A saturated compound is a chemical compound (or ion) that resists addition. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. examples of unsaturated solutions. Apply a solubility conversion. Unsaturated Examples Chemistry.
From www.researchgate.net
Examples of α,βunsaturated compounds in natural products and... Download Scientific Diagram Unsaturated Examples Chemistry Examples of unsaturated organic molecules include hc=ch and h 2 c=o. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. examples of unsaturated solutions. . Unsaturated Examples Chemistry.
From www.pinterest.com
Unsaturated Solution Easy Science Easy science, Solutions, Science student Unsaturated Examples Chemistry an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. A saturated compound is a chemical compound. Unsaturated Examples Chemistry.
From www.slideserve.com
PPT Organic Chemistry Topic 11 Hw P 197 Q 1to18 PowerPoint Presentation ID5515301 Unsaturated Examples Chemistry an unsaturated solution is more dilute than a saturated solution. saturated and unsaturated compounds. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. an unsaturated solution is a solution that contains less. Unsaturated Examples Chemistry.
From education-portal.com
Unsaturated Hydrocarbon Definition & Examples Video & Lesson Transcript Unsaturated Examples Chemistry unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. examples of unsaturated solutions. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable. Unsaturated Examples Chemistry.
From www.youtube.com
F.2.4 Describe the process of hydrogenation of unsaturated fats. YouTube Unsaturated Examples Chemistry examples of unsaturated solutions. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. saturated and unsaturated compounds. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable. Unsaturated Examples Chemistry.
From www.researchgate.net
Representative chemical structure of saturated and unsaturated fatty... Download Scientific Unsaturated Examples Chemistry an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. saturated and unsaturated compounds. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. Adding a spoonful of. Unsaturated Examples Chemistry.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID5525521 Unsaturated Examples Chemistry unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. A saturated compound is a chemical compound (or ion) that resists addition. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. an unsaturated solution is more dilute. Unsaturated Examples Chemistry.
From education-portal.com
Unsaturated Hydrocarbon Definition & Examples Video & Lesson Transcript Unsaturated Examples Chemistry Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of. unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar. Unsaturated Examples Chemistry.
From studylib.net
Unsaturated hydrocarbons Unsaturated Examples Chemistry Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. examples of unsaturated solutions. saturated and unsaturated compounds. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. an unsaturated solution is. Unsaturated Examples Chemistry.
From www.slideserve.com
PPT Unit 7 Solution Chemistry Chapter 13 PowerPoint Presentation, free download ID2680575 Unsaturated Examples Chemistry Examples of unsaturated organic molecules include hc=ch and h 2 c=o. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. an unsaturated solution is more dilute than. Unsaturated Examples Chemistry.
From byjus.com
Fats The difference between saturated and unsaturated fats Byju's Unsaturated Examples Chemistry A saturated compound is a chemical compound (or ion) that resists addition. unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. an unsaturated solution is more dilute than a saturated solution. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. Apply. Unsaturated Examples Chemistry.
From www.ruled.me
Unsaturated Fat What it is & Examples Guide] Unsaturated Examples Chemistry any solution with a concentration of solute below the saturation point of the solute is an unsaturated. A saturated compound is a chemical compound (or ion) that resists addition. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. saturated and unsaturated compounds. Apply a solubility conversion factor to calculate the amount of solute that can be. Unsaturated Examples Chemistry.
From www.pinterest.com
saturated unsaturated and supersaturated solutions Google Search Solubility, How to memorize Unsaturated Examples Chemistry A saturated compound is a chemical compound (or ion) that resists addition. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. Apply a solubility conversion factor to calculate the amount of solute that can be. Unsaturated Examples Chemistry.
From www.researchgate.net
Examples of α,βunsaturated aldehydes, ketones and nitro compounds that... Download Scientific Unsaturated Examples Chemistry examples of unsaturated solutions. unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. A saturated compound is a chemical compound (or ion) that resists addition. any solution with a concentration of solute below the saturation point of the solute is an unsaturated.. Unsaturated Examples Chemistry.
From www.slideshare.net
Unsaturated Carbonyl Compound Unsaturated Examples Chemistry Examples of unsaturated organic molecules include hc=ch and h 2 c=o. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. an unsaturated solution is more dilute than a saturated solution. examples of unsaturated solutions. an unsaturated solution is a solution that contains less than the maximum amount of. Unsaturated Examples Chemistry.
From classnotes.ng
Unsaturated Hydrocarbon Alkenes ClassNotes.ng Unsaturated Examples Chemistry A saturated compound is a chemical compound (or ion) that resists addition. unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. saturated and unsaturated compounds. Examples of unsaturated organic molecules include hc=ch and h 2 c=o. an unsaturated solution is a solution. Unsaturated Examples Chemistry.
From www.thoughtco.com
Unsaturated Definition in Chemistry Unsaturated Examples Chemistry Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. examples of unsaturated solutions. saturated and unsaturated compounds. A saturated compound is a chemical compound (or ion). Unsaturated Examples Chemistry.
From www.youtube.com
SATURATED AND UNSATURATED CARBON COMPOUNDS YouTube Unsaturated Examples Chemistry A saturated compound is a chemical compound (or ion) that resists addition. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. examples of unsaturated solutions. an unsaturated solution is more dilute than a saturated solution. an unsaturated solution is a solution that contains less than the maximum amount of solute. Unsaturated Examples Chemistry.
From www.slideserve.com
PPT Unsaturated Hydrocarbons PowerPoint Presentation, free download ID2168099 Unsaturated Examples Chemistry unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature. any solution with a concentration of solute below the saturation point of the solute is an unsaturated. an unsaturated solution is a solution that contains less than the maximum amount of solute that. Unsaturated Examples Chemistry.