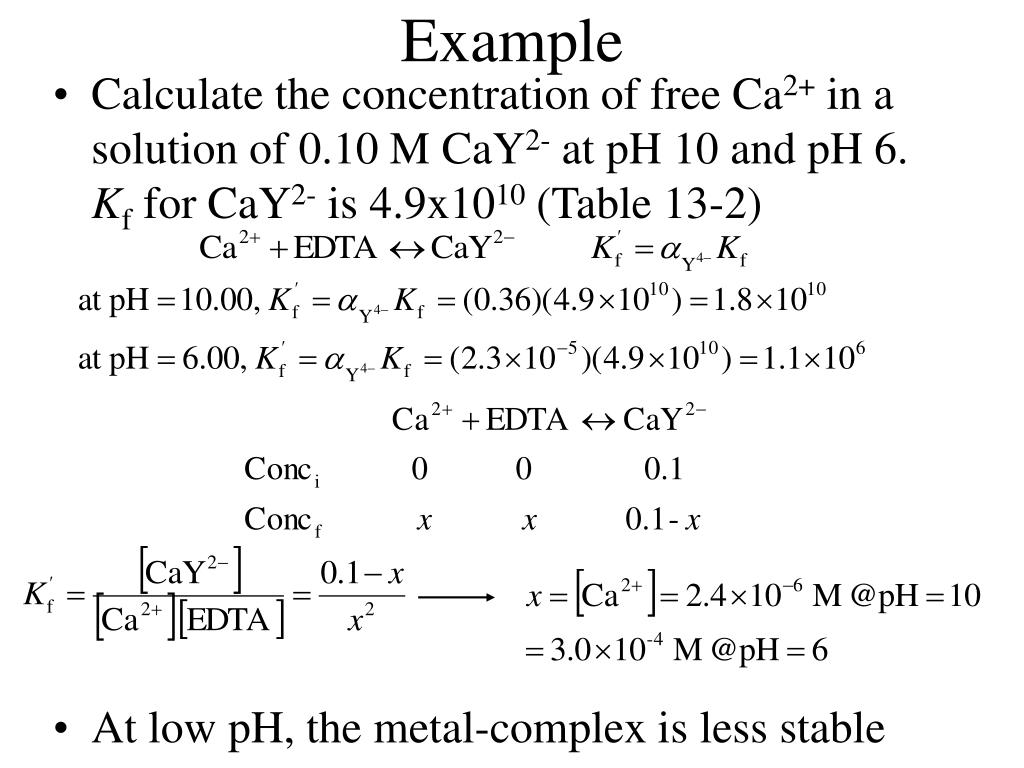
Edta Titration Ph Range . Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. Most edta titrations are performed at ph >= 10. Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b). A solution containing both ca 2+ and fe 3+. All edta equilibrium calculations will use k'f at the ph of the titration. Describe and explain the concept of complex equilibria and stepwise. When the titration is complete,. It is a chelating ligand with two nitrogen and four. (a) titration of 50.0 ml of 0.010 m. You would like to perform a titration of 50.00. Ph for edta titration of fe 3+ is slightly higher than 1.00.
from www.slideserve.com
Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. Describe and explain the concept of complex equilibria and stepwise. You would like to perform a titration of 50.00. Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. When the titration is complete,. Ph for edta titration of fe 3+ is slightly higher than 1.00. Most edta titrations are performed at ph >= 10. All edta equilibrium calculations will use k'f at the ph of the titration. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. (a) titration of 50.0 ml of 0.010 m.
PPT EDTA Titrations PowerPoint Presentation, free download ID234018
Edta Titration Ph Range It is a chelating ligand with two nitrogen and four. All edta equilibrium calculations will use k'f at the ph of the titration. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. Most edta titrations are performed at ph >= 10. Ph for edta titration of fe 3+ is slightly higher than 1.00. When the titration is complete,. If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b). (a) titration of 50.0 ml of 0.010 m. Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. A solution containing both ca 2+ and fe 3+. Describe and explain the concept of complex equilibria and stepwise. It is a chelating ligand with two nitrogen and four. You would like to perform a titration of 50.00.
From www.chegg.com
Solved Speciation of EDTA as a Function of pH 1.2 1 EDTA4 Edta Titration Ph Range Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. You would like to perform a titration of 50.00. If we adjust the ph to. Edta Titration Ph Range.
From www.youtube.com
Total Water Hardness using EDTA Titration YouTube Edta Titration Ph Range (a) titration of 50.0 ml of 0.010 m. When the titration is complete,. A solution containing both ca 2+ and fe 3+. You would like to perform a titration of 50.00. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. Titration curves illustrating how we can use the titrand’s ph to control. Edta Titration Ph Range.
From www.slideserve.com
PPT EDTA Titrations Chapter 13 PowerPoint Presentation, free download Edta Titration Ph Range (a) titration of 50.0 ml of 0.010 m. It is a chelating ligand with two nitrogen and four. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. Ph for edta titration of fe 3+ is slightly higher than 1.00. If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating. Edta Titration Ph Range.
From www.slideserve.com
PPT Chapter 13 EDTA Titrations PowerPoint Presentation, free download Edta Titration Ph Range All edta equilibrium calculations will use k'f at the ph of the titration. Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. Most edta titrations are performed at ph >= 10. A solution containing both ca 2+ and fe 3+. Describe and explain the concept of complex equilibria and stepwise. Edta (ethylene diamine tetraacetic acid). Edta Titration Ph Range.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID89337 Edta Titration Ph Range It is a chelating ligand with two nitrogen and four. A solution containing both ca 2+ and fe 3+. When the titration is complete,. You would like to perform a titration of 50.00. All edta equilibrium calculations will use k'f at the ph of the titration. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in. Edta Titration Ph Range.
From www.slideserve.com
PPT Ch 13 EDTA Titrations PowerPoint Presentation, free download Edta Titration Ph Range Describe and explain the concept of complex equilibria and stepwise. Most edta titrations are performed at ph >= 10. A solution containing both ca 2+ and fe 3+. You would like to perform a titration of 50.00. If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b). Both solutions. Edta Titration Ph Range.
From www.researchgate.net
Fe(II)EDTA concentration variation at different pH values in the range Edta Titration Ph Range Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. You would like to perform a titration of 50.00. Describe and explain the concept of complex equilibria and stepwise. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. Most edta titrations are performed at ph >= 10. All. Edta Titration Ph Range.
From www.researchgate.net
Fe(II)EDTA concentration variation at different pH values in the range Edta Titration Ph Range If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b). Most edta titrations are performed at ph >= 10. It is a chelating ligand with two nitrogen and four. You would like to perform a titration of 50.00. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is. Edta Titration Ph Range.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Edta Titration Ph Range It is a chelating ligand with two nitrogen and four. When the titration is complete,. (a) titration of 50.0 ml of 0.010 m. Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. Most edta titrations are performed at ph >= 10. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in. Edta Titration Ph Range.
From www.youtube.com
Modelling an EDTA Titration Curve YouTube Edta Titration Ph Range Most edta titrations are performed at ph >= 10. It is a chelating ligand with two nitrogen and four. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b). Ph for edta. Edta Titration Ph Range.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Edta Titration Ph Range A solution containing both ca 2+ and fe 3+. If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b). Most edta titrations are performed at ph >= 10. (a) titration of 50.0 ml of 0.010 m. Describe and explain the concept of complex equilibria and stepwise. When the titration. Edta Titration Ph Range.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID234018 Edta Titration Ph Range When the titration is complete,. A solution containing both ca 2+ and fe 3+. If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b). Most edta titrations are performed at ph >= 10. Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. You would. Edta Titration Ph Range.
From www.youtube.com
Chapter 12 EDTA Titration Curves CHM 214 121 YouTube Edta Titration Ph Range (a) titration of 50.0 ml of 0.010 m. Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b).. Edta Titration Ph Range.
From www.slideserve.com
PPT Chapter 12 EDTA Titrations PowerPoint Presentation, free Edta Titration Ph Range It is a chelating ligand with two nitrogen and four. All edta equilibrium calculations will use k'f at the ph of the titration. You would like to perform a titration of 50.00. Describe and explain the concept of complex equilibria and stepwise. When the titration is complete,. Most edta titrations are performed at ph >= 10. Ph for edta titration. Edta Titration Ph Range.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Edta Titration Ph Range Ph for edta titration of fe 3+ is slightly higher than 1.00. If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b). Describe and explain the concept of complex equilibria and stepwise. A solution containing both ca 2+ and fe 3+. You would like to perform a titration of. Edta Titration Ph Range.
From www.chegg.com
Solved 1212. Effect of pH on the EDTA titration. Use Edta Titration Ph Range (a) titration of 50.0 ml of 0.010 m. It is a chelating ligand with two nitrogen and four. When the titration is complete,. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. A solution containing both ca 2+ and fe 3+.. Edta Titration Ph Range.
From www.slideserve.com
PPT Chapter 12 EDTA Titrations PowerPoint Presentation, free Edta Titration Ph Range If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b). It is a chelating ligand with two nitrogen and four. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. A solution containing both ca 2+ and fe 3+. Titration curves illustrating. Edta Titration Ph Range.
From www.clutchprep.com
EDTA Analytical Chemistry Video Clutch Prep Edta Titration Ph Range Most edta titrations are performed at ph >= 10. You would like to perform a titration of 50.00. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. Both solutions are buffered to a ph of 10.0 using a. Edta Titration Ph Range.
From www.slideserve.com
PPT Chapter 12 EDTA Titrations PowerPoint Presentation, free Edta Titration Ph Range (a) titration of 50.0 ml of 0.010 m. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. Describe and explain the concept of complex equilibria and stepwise. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. You would like to perform a titration of 50.00. It is. Edta Titration Ph Range.
From www.slideserve.com
PPT Chapter 12 EDTA Titrations PowerPoint Presentation, free Edta Titration Ph Range It is a chelating ligand with two nitrogen and four. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. You would like to perform a titration of 50.00. Ph for edta titration of fe 3+ is slightly higher than 1.00. If we adjust the ph to 3 we can titrate ni 2+ with edta without. Edta Titration Ph Range.
From www.slideserve.com
PPT Ch 13 EDTA Titrations PowerPoint Presentation, free download Edta Titration Ph Range Ph for edta titration of fe 3+ is slightly higher than 1.00. Most edta titrations are performed at ph >= 10. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. When the titration is complete,. (a) titration of. Edta Titration Ph Range.
From www.slideserve.com
PPT Types of chemistry PowerPoint Presentation, free download ID Edta Titration Ph Range You would like to perform a titration of 50.00. All edta equilibrium calculations will use k'f at the ph of the titration. If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b). Most edta titrations are performed at ph >= 10. A solution containing both ca 2+ and fe. Edta Titration Ph Range.
From www.researchgate.net
Fraction of EDTA species as a function of pH value. Download Edta Titration Ph Range (a) titration of 50.0 ml of 0.010 m. Describe and explain the concept of complex equilibria and stepwise. Most edta titrations are performed at ph >= 10. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. When the titration is complete,. You would like to perform a titration of 50.00. Ph for edta titration of. Edta Titration Ph Range.
From zigzagsci.com
エチレンジアミン四酢酸(EDTA)の性質とキレート滴定について解説 Edta Titration Ph Range You would like to perform a titration of 50.00. When the titration is complete,. If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b). Describe and explain the concept of complex equilibria and stepwise. A solution containing both ca 2+ and fe 3+. Edta (ethylene diamine tetraacetic acid) is. Edta Titration Ph Range.
From www.researchgate.net
In vitro affinity of EDTA for metal ions. The curve represents the Edta Titration Ph Range You would like to perform a titration of 50.00. All edta equilibrium calculations will use k'f at the ph of the titration. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. If we adjust the ph to 3. Edta Titration Ph Range.
From www.slideserve.com
PPT Chapter 12 EDTA Titrations PowerPoint Presentation, free Edta Titration Ph Range Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. Ph for edta titration of fe 3+ is slightly higher than 1.00. If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b). Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia. Edta Titration Ph Range.
From chempedia.info
Equivalence point EDTA titrations Big Chemical Encyclopedia Edta Titration Ph Range Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. All edta equilibrium calculations will use k'f at the ph of the titration. You would like to perform a titration of 50.00. Titration curves illustrating how we can use. Edta Titration Ph Range.
From slideplayer.com
Chapter 11 EDTA Titrations ppt download Edta Titration Ph Range Most edta titrations are performed at ph >= 10. (a) titration of 50.0 ml of 0.010 m. Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. A solution containing both ca 2+ and fe 3+. It is a chelating ligand with. Edta Titration Ph Range.
From www.slideserve.com
PPT Chapter 12 EDTA Titrations PowerPoint Presentation, free Edta Titration Ph Range Ph for edta titration of fe 3+ is slightly higher than 1.00. You would like to perform a titration of 50.00. Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. All edta equilibrium calculations will use k'f at the ph of. Edta Titration Ph Range.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Edta Titration Ph Range Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. (a) titration of 50.0 ml of 0.010 m. All edta equilibrium calculations will use k'f at the ph of the titration. If we adjust the ph to 3 we can titrate ni. Edta Titration Ph Range.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID234018 Edta Titration Ph Range Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. Describe and explain the concept of complex equilibria and stepwise. Ph for edta titration of fe 3+ is slightly higher than 1.00. You would like to perform a titration of 50.00. If we adjust the ph to 3 we can titrate ni 2+. Edta Titration Ph Range.
From www.slideserve.com
PPT EDTA Titrations Chapter 13 PowerPoint Presentation, free download Edta Titration Ph Range When the titration is complete,. Describe and explain the concept of complex equilibria and stepwise. If we adjust the ph to 3 we can titrate ni 2+ with edta without titrating ca 2 + (figure 9.34b). Most edta titrations are performed at ph >= 10. Ph for edta titration of fe 3+ is slightly higher than 1.00. It is a. Edta Titration Ph Range.
From mmerevise.co.uk
pH Curves Questions and Revision MME Edta Titration Ph Range Describe and explain the concept of complex equilibria and stepwise. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the complexometric titration. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. It is a chelating ligand with two nitrogen and four. A solution containing both ca 2+ and fe 3+.. Edta Titration Ph Range.
From www.slideshare.net
Complexometric titration Edta Titration Ph Range Ph for edta titration of fe 3+ is slightly higher than 1.00. A solution containing both ca 2+ and fe 3+. Both solutions are buffered to a ph of 10.0 using a 0.100m ammonia buffer. Describe and explain the concept of complex equilibria and stepwise. Edta (ethylene diamine tetraacetic acid) is an organic reagent that is frequently used in the. Edta Titration Ph Range.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Edta Titration Ph Range When the titration is complete,. All edta equilibrium calculations will use k'f at the ph of the titration. Most edta titrations are performed at ph >= 10. Titration curves illustrating how we can use the titrand’s ph to control edta’s selectivity. Describe and explain the concept of complex equilibria and stepwise. You would like to perform a titration of 50.00.. Edta Titration Ph Range.