Iodine Titration Are Usually Used With . To know when the reaction is complete, we use starch solution as an indicator. Iodometry and iodimetry are titration techniques used to determine the concentration of unknown solutions by using redox reactions. The titration can be performed using just iodine solution and not iodate, but the iodate solution is more stable and gives a more accurate result. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. In iodimetric titrations, the analyte (a. Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a sample. The main difference between the two is the role of. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing agents. The vitamin c titration procedure is appropriate for testing the amount of vitamin c in tablets, juices, and fresh or frozen fruits and vegetables. Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent.
from slidetodoc.com
Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a sample. To know when the reaction is complete, we use starch solution as an indicator. The main difference between the two is the role of. Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing agents. Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. The vitamin c titration procedure is appropriate for testing the amount of vitamin c in tablets, juices, and fresh or frozen fruits and vegetables. Iodometry and iodimetry are titration techniques used to determine the concentration of unknown solutions by using redox reactions. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. In iodimetric titrations, the analyte (a.
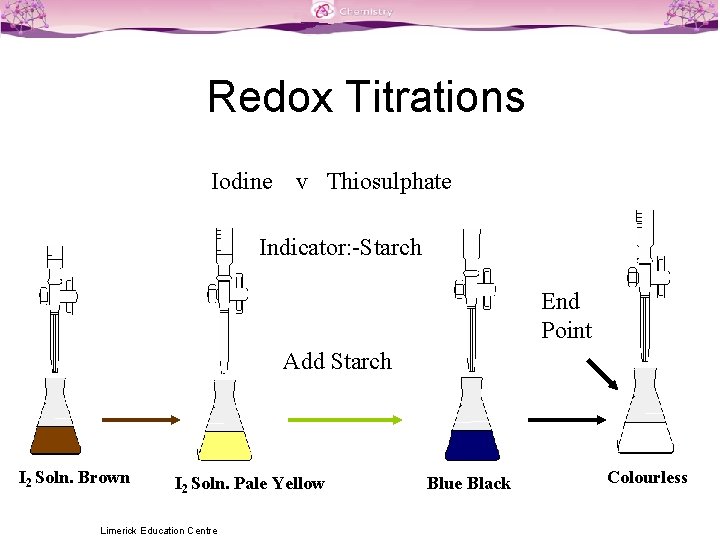
Titration Colour Changes SLSS Science Limerick Education Centre
Iodine Titration Are Usually Used With The vitamin c titration procedure is appropriate for testing the amount of vitamin c in tablets, juices, and fresh or frozen fruits and vegetables. The vitamin c titration procedure is appropriate for testing the amount of vitamin c in tablets, juices, and fresh or frozen fruits and vegetables. The main difference between the two is the role of. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a sample. To know when the reaction is complete, we use starch solution as an indicator. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. The titration can be performed using just iodine solution and not iodate, but the iodate solution is more stable and gives a more accurate result. Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing agents. In iodimetric titrations, the analyte (a. Iodometry and iodimetry are titration techniques used to determine the concentration of unknown solutions by using redox reactions.
From www.youtube.com
Titration Involves Iodine (Iodimetry) Redox Titration Iodine Titration Are Usually Used With In iodimetric titrations, the analyte (a. The vitamin c titration procedure is appropriate for testing the amount of vitamin c in tablets, juices, and fresh or frozen fruits and vegetables. The main difference between the two is the role of. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Titrations involving iodine. Iodine Titration Are Usually Used With.
From www.sciencephoto.com
End point of an iodine titration. 3 of 5. Stock Image C029/1102 Iodine Titration Are Usually Used With Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a sample. Iodometry and iodimetry are titration techniques used to determine the concentration of unknown solutions by using redox reactions. In iodimetric titrations, the analyte (a. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry. Iodine Titration Are Usually Used With.
From www.scribd.com
Titration of Iodine With Standard Thisulphate Solution Titration Iodine Iodine Titration Are Usually Used With Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a sample. Iodometry and iodimetry are titration techniques used to determine the concentration of unknown solutions by using redox reactions. The vitamin c titration procedure is appropriate for testing the amount of vitamin c in tablets, juices, and fresh or frozen fruits. Iodine Titration Are Usually Used With.
From www.numerade.com
SOLVED The titration of SO2 by iodine in the presence of starch is Iodine Titration Are Usually Used With Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing agents. The titration can be performed using just iodine solution and not iodate, but the iodate solution is more stable and gives a more accurate result. It works. Iodine Titration Are Usually Used With.
From studylib.net
Salt Iodine Titration Method Iodine Titration Are Usually Used With In iodimetric titrations, the analyte (a. Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing agents. Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. The vitamin c titration procedure. Iodine Titration Are Usually Used With.
From www.scribd.com
Iodometrictitrationprepofhypoiodine_jaya06.02.18 PDF Iodine Titration Are Usually Used With Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. In iodimetric titrations, the analyte (a. The main difference between the two is the role of. Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. The titration can be performed using just iodine solution and not. Iodine Titration Are Usually Used With.
From www.numerade.com
SOLVED Vitamin C (ascorbic acid, C6H8O6, 176.12 g / mol ) can be Iodine Titration Are Usually Used With Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing agents. The vitamin c titration procedure is appropriate for testing the amount of vitamin c in tablets, juices, and fresh or frozen fruits and vegetables. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Iodometry and iodimetry are. Iodine Titration Are Usually Used With.
From themasterchemistry.com
Iodometric Titration Principle, Example, Advantages Iodine Titration Are Usually Used With Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a sample. Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing agents. Iodometry and iodimetry are titration techniques. Iodine Titration Are Usually Used With.
From www.slideserve.com
PPT Real World Vitamin C Concentration and Total Acidity in Orange Iodine Titration Are Usually Used With Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a sample. To know when the reaction is complete, we use starch solution as an indicator. The main difference between the two is the role of. Iodometry and iodimetry are titration techniques used to determine the concentration of unknown solutions by using. Iodine Titration Are Usually Used With.
From www.scribd.com
05. Titration Iodine Titration Iodine Titration Are Usually Used With Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. The titration can be performed using just iodine solution and not iodate, but the iodate solution is more stable and gives a more accurate result. Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a. Iodine Titration Are Usually Used With.
From www.sciencephoto.com
End point of an iodine titration. 5 of 5. Stock Image C029/1118 Iodine Titration Are Usually Used With The vitamin c titration procedure is appropriate for testing the amount of vitamin c in tablets, juices, and fresh or frozen fruits and vegetables. Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a sample. In iodimetric titrations, the analyte (a. The titration can be performed using just iodine solution and. Iodine Titration Are Usually Used With.
From www.scribd.com
Vitaminc Iodine Titration Titration Vitamin C Iodine Titration Are Usually Used With In iodimetric titrations, the analyte (a. Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing agents. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Iodometry titration is a. Iodine Titration Are Usually Used With.
From theedge.com.hk
Chemistry How To Titration The Edge Iodine Titration Are Usually Used With To know when the reaction is complete, we use starch solution as an indicator. The titration can be performed using just iodine solution and not iodate, but the iodate solution is more stable and gives a more accurate result. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Iodometry titration is. Iodine Titration Are Usually Used With.
From www.sciencephoto.com
Iodine and thiosulfate ions titration Stock Image C033/2858 Iodine Titration Are Usually Used With Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. The main difference between the two is the role of. The titration can be performed using just iodine solution and not iodate, but the iodate solution is more stable and gives a more accurate result. Iodometric titration is a method used to. Iodine Titration Are Usually Used With.
From www.researchgate.net
Protocol schematic showing the iodine titration diet progression at the Iodine Titration Are Usually Used With Iodometry and iodimetry are titration techniques used to determine the concentration of unknown solutions by using redox reactions. In iodimetric titrations, the analyte (a. The main difference between the two is the role of. Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. Iodometry titration is a volumetric analysis method that is used. Iodine Titration Are Usually Used With.
From www.sciencephoto.com
End point of an iodine titration. 2 of 5. Stock Image C029/1115 Iodine Titration Are Usually Used With To know when the reaction is complete, we use starch solution as an indicator. The titration can be performed using just iodine solution and not iodate, but the iodate solution is more stable and gives a more accurate result. Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a sample. Iodometric. Iodine Titration Are Usually Used With.
From www.youtube.com
Iodine Titration Set YouTube Iodine Titration Are Usually Used With The main difference between the two is the role of. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. The titration can be performed using just iodine solution and not iodate, but the iodate solution. Iodine Titration Are Usually Used With.
From www.youtube.com
Iodine Titration Set YouTube Iodine Titration Are Usually Used With To know when the reaction is complete, we use starch solution as an indicator. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. In iodimetric titrations, the analyte (a. The main difference between the two is the role of. It works by mixing the oxidizing agent with iodide ions, which causes iodine. Iodine Titration Are Usually Used With.
From www.researchgate.net
Titrating method with iodine solution Download Scientific Diagram Iodine Titration Are Usually Used With Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. The main difference between the two is the role of. Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing agents.. Iodine Titration Are Usually Used With.
From www.researchgate.net
Left peptide solution during iodine titration showing the solution Iodine Titration Are Usually Used With It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. To know when the reaction is complete, we use starch solution as an indicator. Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. The main difference between the two is the role of. Titrations involving iodine have. Iodine Titration Are Usually Used With.
From present5.com
Dissolved Oxygen and Biochemical Oxygen Demand Analyses Prepared Iodine Titration Are Usually Used With It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. The vitamin c titration procedure is appropriate for testing the amount of vitamin c in tablets, juices, and fresh or frozen fruits and vegetables. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Iodometry and. Iodine Titration Are Usually Used With.
From fphoto.photoshelter.com
science chemistry titration iodine thiosulfate Fundamental Iodine Titration Are Usually Used With Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. To know when the reaction is complete, we use starch solution as an indicator. In iodimetric titrations, the analyte (a. The main difference between the two is the role of. It works by mixing the oxidizing agent with iodide ions, which causes iodine. Iodine Titration Are Usually Used With.
From www.scribd.com
Iodine Titrations PDF Titration Chemistry Iodine Titration Are Usually Used With The titration can be performed using just iodine solution and not iodate, but the iodate solution is more stable and gives a more accurate result. The main difference between the two is the role of. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Iodometry titration is a volumetric analysis method that is. Iodine Titration Are Usually Used With.
From www.alamy.com
Finding the end point of an iodine titration. At the start of an iodine Iodine Titration Are Usually Used With The titration can be performed using just iodine solution and not iodate, but the iodate solution is more stable and gives a more accurate result. Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a sample. Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing. Iodine Titration Are Usually Used With.
From www.alamy.com
Finding the end point of an iodine titration. 1 of 5. At the start of Iodine Titration Are Usually Used With Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing agents. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. Iodometric titration is a method used to measure the amount. Iodine Titration Are Usually Used With.
From www.youtube.com
Iodine / Thiosulfate Redox Titration Demonstration YouTube Iodine Titration Are Usually Used With Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a sample. Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing agents. Iodometry and iodimetry are titration techniques used to determine the concentration of unknown solutions by using redox reactions. Iodometry and iodimetry are two different. Iodine Titration Are Usually Used With.
From www.youtube.com
KAC31.4 Titrations II (Redox) Iodine/Thiosulfate Titrations YouTube Iodine Titration Are Usually Used With It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing agents. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. To know when the reaction is complete, we use starch. Iodine Titration Are Usually Used With.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Iodine Titration Are Usually Used With Iodometry and iodimetry are titration techniques used to determine the concentration of unknown solutions by using redox reactions. Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a sample. It works by mixing the oxidizing. Iodine Titration Are Usually Used With.
From slidetodoc.com
Experiment three Assay test of Povidone Iodine solution Iodine Titration Are Usually Used With Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. The main difference between the two is the role of. In iodimetric titrations, the analyte (a. To know when the reaction is complete, we use starch solution as an indicator. Iodometry and iodimetry are titration techniques used to determine the concentration of unknown solutions. Iodine Titration Are Usually Used With.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Iodine Titration Are Usually Used With Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. The vitamin c titration procedure is appropriate for testing the amount of vitamin c in tablets, juices, and fresh or frozen fruits and vegetables. Thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Iodometry and. Iodine Titration Are Usually Used With.
From byjus.com
Na thiosulfate in iodine titration methods Iodine Titration Are Usually Used With It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. The main difference between the two is the role of. Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. Iodometry and iodimetry are titration techniques used to determine the concentration of unknown solutions by using redox reactions.. Iodine Titration Are Usually Used With.
From www.youtube.com
Redox titration Part 3_Iodine titration_Iodometry and Iodimetry Iodine Titration Are Usually Used With Titrations involving iodine have evolved for the analysis of a number of oxidizing and reducing agents. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. The titration can be performed using just iodine solution and not iodate, but the iodate solution is more stable and gives a more accurate result. To know when. Iodine Titration Are Usually Used With.
From www.youtube.com
Redox TitrationIodine solution & Sodium Thiosulphate (Na2S2O3) WAEC Iodine Titration Are Usually Used With Iodometry and iodimetry are titration techniques used to determine the concentration of unknown solutions by using redox reactions. The main difference between the two is the role of. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. In iodimetric titrations, the analyte (a. Iodometry titration is a volumetric analysis method that is. Iodine Titration Are Usually Used With.
From www.vernier.com
Potentiometric Titration of Aqueous Iodine Iodine Titration Are Usually Used With Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Iodometry titration is a volumetric analysis method that is used to determine the amount of iodine present in a sample. Iodometry and iodimetry are titration techniques used to determine the concentration of unknown solutions by using redox reactions. Titrations involving iodine have evolved. Iodine Titration Are Usually Used With.
From studylib.net
An iodine / thiosulfate titration Iodine Titration Are Usually Used With It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Iodometry and iodimetry are two different types of titration methods that use iodine as a reagent. Iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. To know when the reaction is complete, we use starch solution. Iodine Titration Are Usually Used With.