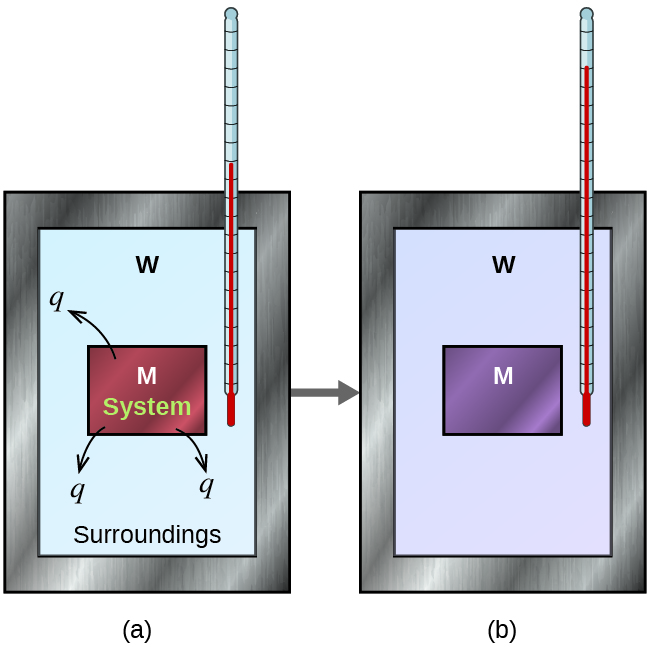
Is Calorimeter Constant Positive Or Negative . Q is given a positive (+) sign when the system absorbs heat from the. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. A positive δh means that heat flows into a system from its. Try performing multiple trials and averaging out the results of those trials to reduce. In the specific situation described, q substance m is a negative value and q substance w is positive, since heat is transferred from m to w. If δt and q are positive, then. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal. A negative δh means that heat flows from a system to its surroundings; How to calculate a calorimeter constant. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a.
from wisc.pb.unizin.org
If δt and q are positive, then. Q is given a positive (+) sign when the system absorbs heat from the. A negative δh means that heat flows from a system to its surroundings; How to calculate a calorimeter constant. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Apply the first law of thermodynamics to calorimetry. A positive δh means that heat flows into a system from its. Try performing multiple trials and averaging out the results of those trials to reduce. In the specific situation described, q substance m is a negative value and q substance w is positive, since heat is transferred from m to w. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign.
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow
Is Calorimeter Constant Positive Or Negative Q is given a positive (+) sign when the system absorbs heat from the. Q is given a positive (+) sign when the system absorbs heat from the. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. If δt and q are positive, then. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Compare heat flow from hot to cold objects in an ideal. A positive δh means that heat flows into a system from its. A negative δh means that heat flows from a system to its surroundings; How to calculate a calorimeter constant. Apply the first law of thermodynamics to calorimetry. In the specific situation described, q substance m is a negative value and q substance w is positive, since heat is transferred from m to w. Try performing multiple trials and averaging out the results of those trials to reduce.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Is Calorimeter Constant Positive Or Negative Apply the first law of thermodynamics to calorimetry. In the specific situation described, q substance m is a negative value and q substance w is positive, since heat is transferred from m to w. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Try performing multiple trials and averaging. Is Calorimeter Constant Positive Or Negative.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Is Calorimeter Constant Positive Or Negative If δt and q are positive, then. Apply the first law of thermodynamics to calorimetry. Try performing multiple trials and averaging out the results of those trials to reduce. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. Q is given a. Is Calorimeter Constant Positive Or Negative.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Is Calorimeter Constant Positive Or Negative If δt and q are positive, then. A positive δh means that heat flows into a system from its. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Q is given a positive (+) sign when the system absorbs heat from the. How to calculate a calorimeter constant. Try. Is Calorimeter Constant Positive Or Negative.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Is Calorimeter Constant Positive Or Negative Q is given a positive (+) sign when the system absorbs heat from the. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. In the specific situation described, q substance m is a negative value and q substance w is positive, since. Is Calorimeter Constant Positive Or Negative.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Is Calorimeter Constant Positive Or Negative In the specific situation described, q substance m is a negative value and q substance w is positive, since heat is transferred from m to w. Apply the first law of thermodynamics to calorimetry. A positive δh means that heat flows into a system from its. A negative δh means that heat flows from a system to its surroundings; Try. Is Calorimeter Constant Positive Or Negative.
From www.youtube.com
050 Calorimetry YouTube Is Calorimeter Constant Positive Or Negative A negative δh means that heat flows from a system to its surroundings; Apply the first law of thermodynamics to calorimetry. A positive δh means that heat flows into a system from its. Q is given a positive (+) sign when the system absorbs heat from the. When 40.0 ml of water at 60.0 °c is added to 40.0 ml. Is Calorimeter Constant Positive Or Negative.
From slideplayer.com
Calorimetry Chapter ppt download Is Calorimeter Constant Positive Or Negative Compare heat flow from hot to cold objects in an ideal. Q is given a positive (+) sign when the system absorbs heat from the. If δt and q are positive, then. How to calculate a calorimeter constant. A positive δh means that heat flows into a system from its. Try performing multiple trials and averaging out the results of. Is Calorimeter Constant Positive Or Negative.
From www.chegg.com
Solved Thermometer A bomb calorimeter, or constant volume Is Calorimeter Constant Positive Or Negative The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. In the specific situation described, q substance m is a negative value and q substance w is positive, since heat is transferred from m to w. A negative δh means that heat flows. Is Calorimeter Constant Positive Or Negative.
From www.slideserve.com
PPT Zumdahl Chapter 6 PowerPoint Presentation, free download ID4269919 Is Calorimeter Constant Positive Or Negative The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. In the specific situation described, q substance m is a negative value and q substance w is positive, since heat is transferred from m to w. Q is given a positive (+) sign. Is Calorimeter Constant Positive Or Negative.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Is Calorimeter Constant Positive Or Negative Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal. In the specific situation described, q substance m is a negative value and q substance w is positive, since heat is transferred from m to w. A positive δh means that heat flows into a system from its. Try performing multiple. Is Calorimeter Constant Positive Or Negative.
From users.highland.edu
Calorimetry Is Calorimeter Constant Positive Or Negative Try performing multiple trials and averaging out the results of those trials to reduce. A positive δh means that heat flows into a system from its. A negative δh means that heat flows from a system to its surroundings; When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. In. Is Calorimeter Constant Positive Or Negative.
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube Is Calorimeter Constant Positive Or Negative Compare heat flow from hot to cold objects in an ideal. Apply the first law of thermodynamics to calorimetry. How to calculate a calorimeter constant. Try performing multiple trials and averaging out the results of those trials to reduce. A positive δh means that heat flows into a system from its. If δt and q are positive, then. In the. Is Calorimeter Constant Positive Or Negative.
From studylib.net
Heat Equation Is Calorimeter Constant Positive Or Negative Compare heat flow from hot to cold objects in an ideal. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. A negative. Is Calorimeter Constant Positive Or Negative.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Is Calorimeter Constant Positive Or Negative Q is given a positive (+) sign when the system absorbs heat from the. If δt and q are positive, then. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. A positive δh means that heat flows into a system from its. Apply the first law of thermodynamics to. Is Calorimeter Constant Positive Or Negative.
From users.highland.edu
Calorimetry Is Calorimeter Constant Positive Or Negative Compare heat flow from hot to cold objects in an ideal. Q is given a positive (+) sign when the system absorbs heat from the. How to calculate a calorimeter constant. Try performing multiple trials and averaging out the results of those trials to reduce. If δt and q are positive, then. When 40.0 ml of water at 60.0 °c. Is Calorimeter Constant Positive Or Negative.
From www.youtube.com
How to find calorimeter constant YouTube Is Calorimeter Constant Positive Or Negative In the specific situation described, q substance m is a negative value and q substance w is positive, since heat is transferred from m to w. A positive δh means that heat flows into a system from its. Try performing multiple trials and averaging out the results of those trials to reduce. Apply the first law of thermodynamics to calorimetry.. Is Calorimeter Constant Positive Or Negative.
From www.thoughtco.com
Calorimeter Definition in Chemistry Is Calorimeter Constant Positive Or Negative In the specific situation described, q substance m is a negative value and q substance w is positive, since heat is transferred from m to w. A positive δh means that heat flows into a system from its. Compare heat flow from hot to cold objects in an ideal. If δt and q are positive, then. Q is given a. Is Calorimeter Constant Positive Or Negative.
From slideplayer.com
Chapter 17 Thermochemistry 17.2 Measuring and Expressing ppt download Is Calorimeter Constant Positive Or Negative A negative δh means that heat flows from a system to its surroundings; Try performing multiple trials and averaging out the results of those trials to reduce. Apply the first law of thermodynamics to calorimetry. If δt and q are positive, then. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already. Is Calorimeter Constant Positive Or Negative.
From www.youtube.com
Principle of Calorimetry YouTube Is Calorimeter Constant Positive Or Negative In the specific situation described, q substance m is a negative value and q substance w is positive, since heat is transferred from m to w. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Apply the first law of thermodynamics to calorimetry. If δt and q are positive,. Is Calorimeter Constant Positive Or Negative.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Is Calorimeter Constant Positive Or Negative The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. In the specific situation described, q substance m is a negative value and q substance w is positive, since heat is transferred from m to w. A negative δh means that heat flows. Is Calorimeter Constant Positive Or Negative.
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter Is Calorimeter Constant Positive Or Negative Q is given a positive (+) sign when the system absorbs heat from the. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Compare heat flow from hot to cold objects in an ideal. How to calculate a calorimeter constant. A negative δh means that heat flows from a. Is Calorimeter Constant Positive Or Negative.
From www.freeastroscience.com
Exploring Calorimetry A Journey into Heat Transfer Measurement Is Calorimeter Constant Positive Or Negative If δt and q are positive, then. How to calculate a calorimeter constant. Compare heat flow from hot to cold objects in an ideal. Q is given a positive (+) sign when the system absorbs heat from the. Try performing multiple trials and averaging out the results of those trials to reduce. When 40.0 ml of water at 60.0 °c. Is Calorimeter Constant Positive Or Negative.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Is Calorimeter Constant Positive Or Negative When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. If δt and q are positive, then. In the specific situation described, q. Is Calorimeter Constant Positive Or Negative.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Is Calorimeter Constant Positive Or Negative Apply the first law of thermodynamics to calorimetry. The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. How to calculate a calorimeter constant. A negative δh means that heat flows from a system to its surroundings; Q is given a positive (+). Is Calorimeter Constant Positive Or Negative.
From slideplayer.com
Calorimetry. ppt download Is Calorimeter Constant Positive Or Negative The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. A negative δh means that heat flows from a system to its surroundings; A positive δh means that heat flows into a system from its. How to calculate a calorimeter constant. Apply the. Is Calorimeter Constant Positive Or Negative.
From www.tessshebaylo.com
Equation For Constant Volume Calorimetry Tessshebaylo Is Calorimeter Constant Positive Or Negative A positive δh means that heat flows into a system from its. Try performing multiple trials and averaging out the results of those trials to reduce. A negative δh means that heat flows from a system to its surroundings; Q is given a positive (+) sign when the system absorbs heat from the. The value of c is intrinsically a. Is Calorimeter Constant Positive Or Negative.
From rumble.com
Calorimetry, Constant Pressure, Thermodynamics Chemistry Is Calorimeter Constant Positive Or Negative Q is given a positive (+) sign when the system absorbs heat from the. How to calculate a calorimeter constant. Apply the first law of thermodynamics to calorimetry. Try performing multiple trials and averaging out the results of those trials to reduce. If δt and q are positive, then. Compare heat flow from hot to cold objects in an ideal.. Is Calorimeter Constant Positive Or Negative.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Is Calorimeter Constant Positive Or Negative Q is given a positive (+) sign when the system absorbs heat from the. How to calculate a calorimeter constant. A negative δh means that heat flows from a system to its surroundings; Try performing multiple trials and averaging out the results of those trials to reduce. In the specific situation described, q substance m is a negative value and. Is Calorimeter Constant Positive Or Negative.
From www.youtube.com
Coffee Cup Calorimeter Calculate Enthalpy Change, Constant Pressure Is Calorimeter Constant Positive Or Negative A positive δh means that heat flows into a system from its. Try performing multiple trials and averaging out the results of those trials to reduce. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Compare heat flow from hot to cold objects in an ideal. In the specific. Is Calorimeter Constant Positive Or Negative.
From www.w3schools.blog
Cp, Cv calorimetry W3schools Is Calorimeter Constant Positive Or Negative A negative δh means that heat flows from a system to its surroundings; Q is given a positive (+) sign when the system absorbs heat from the. Apply the first law of thermodynamics to calorimetry. Try performing multiple trials and averaging out the results of those trials to reduce. A positive δh means that heat flows into a system from. Is Calorimeter Constant Positive Or Negative.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Is Calorimeter Constant Positive Or Negative Apply the first law of thermodynamics to calorimetry. In the specific situation described, q substance m is a negative value and q substance w is positive, since heat is transferred from m to w. A negative δh means that heat flows from a system to its surroundings; Compare heat flow from hot to cold objects in an ideal. A positive. Is Calorimeter Constant Positive Or Negative.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Is Calorimeter Constant Positive Or Negative Apply the first law of thermodynamics to calorimetry. Q is given a positive (+) sign when the system absorbs heat from the. A negative δh means that heat flows from a system to its surroundings; How to calculate a calorimeter constant. The value of c is intrinsically a positive number, but δt and q can be either positive or negative,. Is Calorimeter Constant Positive Or Negative.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab Is Calorimeter Constant Positive Or Negative A positive δh means that heat flows into a system from its. Apply the first law of thermodynamics to calorimetry. Q is given a positive (+) sign when the system absorbs heat from the. Try performing multiple trials and averaging out the results of those trials to reduce. If δt and q are positive, then. How to calculate a calorimeter. Is Calorimeter Constant Positive Or Negative.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Is Calorimeter Constant Positive Or Negative The value of c is intrinsically a positive number, but δt and q can be either positive or negative, and they both must have the same sign. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. A positive δh means that heat flows into a system from its. Try. Is Calorimeter Constant Positive Or Negative.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Is Calorimeter Constant Positive Or Negative Q is given a positive (+) sign when the system absorbs heat from the. A positive δh means that heat flows into a system from its. Compare heat flow from hot to cold objects in an ideal. A negative δh means that heat flows from a system to its surroundings; Apply the first law of thermodynamics to calorimetry. If δt. Is Calorimeter Constant Positive Or Negative.