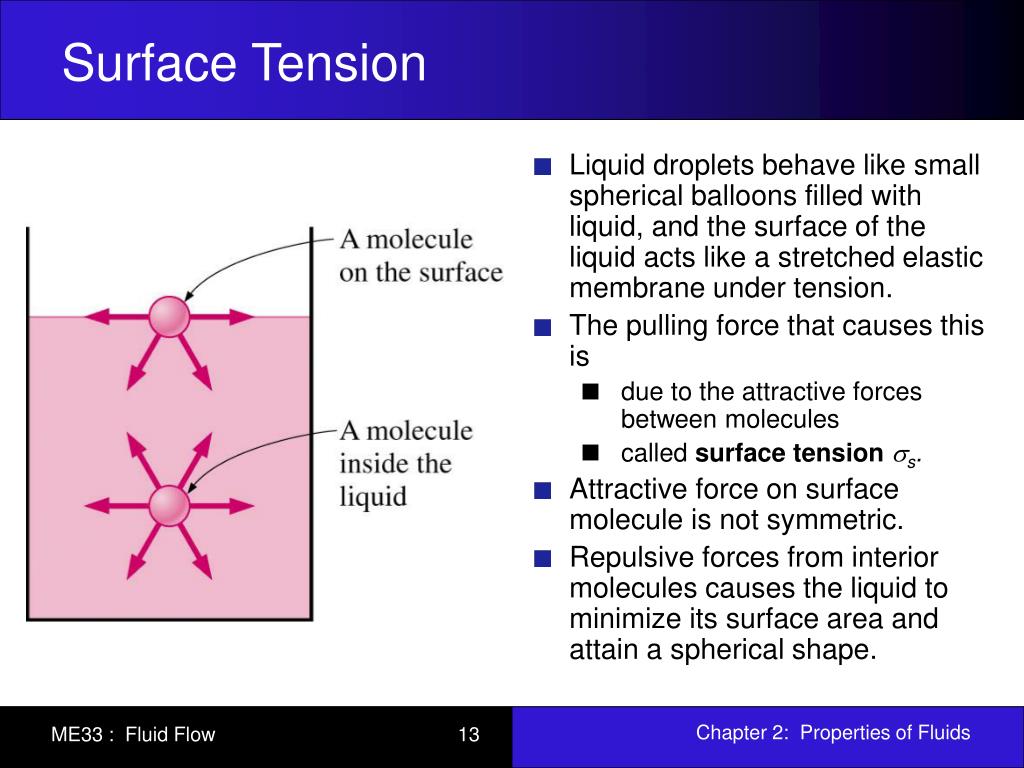
Surface Tension Of Liquid Depends On . Surface tension is the energy required to increase. Surface tension depends on the nature of the liquid, the surrounding. Surface tension is caused by the effects of intermolecular forces at the interface. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which tends to minimise. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. The molecules on the surface of a liquid are attracted by their neighbors from the.
from www.slideserve.com
Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. The molecules on the surface of a liquid are attracted by their neighbors from the. Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy required to increase. Surface tension depends on the nature of the liquid, the surrounding. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which tends to minimise. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions.
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free
Surface Tension Of Liquid Depends On “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which tends to minimise. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension depends on the nature of the liquid, the surrounding. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which tends to minimise. Since these intermolecular forces vary depending on the nature of the. Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Surface tension is the energy required to increase.
From www.youtube.com
Surface Tension on Liquid Jet formula derivation with 3d animation Surface Tension Of Liquid Depends On Surface tension is the energy required to increase. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface of a liquid are attracted by their neighbors from the. Since these intermolecular forces vary depending on the nature of the. “surface tension is the tension of. Surface Tension Of Liquid Depends On.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Surface Tension Of Liquid Depends On Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension is the energy, or work, required to increase the surface area of a liquid due. Surface Tension Of Liquid Depends On.
From engineeringcheatsheet.com
Surface Tension of Liquids Engineering Cheat Sheet Surface Tension Of Liquid Depends On Since these intermolecular forces vary depending on the nature of the. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which. Surface Tension Of Liquid Depends On.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Surface Tension Of Liquid Depends On Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Surface tension depends on the nature of the liquid, the surrounding. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. “surface tension. Surface Tension Of Liquid Depends On.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Surface Tension Of Liquid Depends On Surface tension is the energy required to increase. Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in. Surface Tension Of Liquid Depends On.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation Surface Tension Of Liquid Depends On Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is caused by the. Surface Tension Of Liquid Depends On.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2735913 Surface Tension Of Liquid Depends On Surface tension depends on the nature of the liquid, the surrounding. Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the. Surface Tension Of Liquid Depends On.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Surface Tension Of Liquid Depends On Since these intermolecular forces vary depending on the nature of the. Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of the surface film of a liquid caused by the attraction. Surface Tension Of Liquid Depends On.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Surface Tension Of Liquid Depends On Surface tension is the energy required to increase. Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the. “surface tension is the tension of the surface. Surface Tension Of Liquid Depends On.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Surface Tension Of Liquid Depends On Surface tension is the energy required to increase. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Surface tension depends on the nature of the liquid, the surrounding. Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension is the energy, or work, required to. Surface Tension Of Liquid Depends On.
From www.logiota.com
Surface Tension States of Matter Physical Chemistry Chemistry Surface Tension Of Liquid Depends On Surface tension is the energy required to increase. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Surface tension is a. Surface Tension Of Liquid Depends On.
From www.youtube.com
Surface Tension of Water Explained YouTube Surface Tension Of Liquid Depends On Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required to increase. Surface tension depends on the nature of the liquid, the surrounding. Surface tension is caused by the effects of intermolecular forces. Surface Tension Of Liquid Depends On.
From www.youtube.com
What is Surface Tension Liquid State Liquid Dynamics Surface Surface Tension Of Liquid Depends On Surface tension is the energy required to increase. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which tends to minimise. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular. Surface Tension Of Liquid Depends On.
From www.youtube.com
Chemistry 8.2b Properties of Liquids Surface Tension and Capillary Surface Tension Of Liquid Depends On Surface tension depends on the nature of the liquid, the surrounding. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Surface tension is the energy required to increase. “surface tension is the tension of the surface film of a liquid caused. Surface Tension Of Liquid Depends On.
From www.youtube.com
Why are liquid drops spherical? Surface Tension YouTube Surface Tension Of Liquid Depends On The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the energy required to increase. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which tends to minimise. Surface tension is the. Surface Tension Of Liquid Depends On.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson Surface Tension Of Liquid Depends On Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles. Surface Tension Of Liquid Depends On.
From www.britannica.com
surface tension Definition, Examples, & Facts Surface Tension Of Liquid Depends On Surface tension is caused by the effects of intermolecular forces at the interface. Since these intermolecular forces vary depending on the nature of the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface. Surface Tension Of Liquid Depends On.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Surface Tension Of Liquid Depends On Surface tension is the energy required to increase. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Surface tension depends on the nature of the liquid,. Surface Tension Of Liquid Depends On.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 Surface Tension Of Liquid Depends On Surface tension is caused by the effects of intermolecular forces at the interface. Since these intermolecular forces vary depending on the nature of the. Surface tension depends on the nature of the liquid, the surrounding. Surface tension is the energy required to increase. “surface tension is the tension of the surface film of a liquid caused by the attraction of. Surface Tension Of Liquid Depends On.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension Of Liquid Depends On Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension depends on the nature of the liquid, the surrounding. Surface tension is caused by the effects of intermolecular forces at the interface. The molecules on the surface of a liquid are attracted by their neighbors from the. Since these intermolecular forces vary depending. Surface Tension Of Liquid Depends On.
From slideplayer.com
SURFACE TENSION OF A LIQUID for 1st sem ppt download Surface Tension Of Liquid Depends On Since these intermolecular forces vary depending on the nature of the. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Surface tension. Surface Tension Of Liquid Depends On.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse Surface Tension Of Liquid Depends On Surface tension is the energy required to increase. Since these intermolecular forces vary depending on the nature of the. Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it.. Surface Tension Of Liquid Depends On.
From selfstudypoint.in
Liquid State Surface Tension Of Liquid Depends On Surface tension is caused by the effects of intermolecular forces at the interface. The molecules on the surface of a liquid are attracted by their neighbors from the. Since these intermolecular forces vary depending on the nature of the. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the. Surface Tension Of Liquid Depends On.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free Surface Tension Of Liquid Depends On Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Surface tension is the energy required to increase. The molecules on the surface of a liquid are attracted by their neighbors from the. Since these intermolecular forces vary. Surface Tension Of Liquid Depends On.
From www.vrogue.co
What Are The Importances Of Surface Tension And Visco vrogue.co Surface Tension Of Liquid Depends On Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension depends on the nature of the liquid, the surrounding. Surface. Surface Tension Of Liquid Depends On.
From www.sliderbase.com
Properties of Liquids Presentation Chemistry Surface Tension Of Liquid Depends On Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. “surface tension is the tension of the surface film of a liquid caused by the attraction of. Surface Tension Of Liquid Depends On.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID Surface Tension Of Liquid Depends On Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension depends on the nature of the liquid, the surrounding. Surface. Surface Tension Of Liquid Depends On.
From byjus.com
Time Period T of a small drop of liquid ( due to surface tension Surface Tension Of Liquid Depends On Surface tension is caused by the effects of intermolecular forces at the interface. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which tends to minimise. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid. Surface Tension Of Liquid Depends On.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID6036532 Surface Tension Of Liquid Depends On Since these intermolecular forces vary depending on the nature of the. Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Surface tension, capillary action, and viscosity are unique. Surface Tension Of Liquid Depends On.
From www.meritnation.com
Surface tension acting on the liquid depends upon mass of the liquid Surface Tension Of Liquid Depends On Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Surface tension depends on the nature of the liquid, the surrounding. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Surface tension. Surface Tension Of Liquid Depends On.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Surface Tension Of Liquid Depends On Surface tension is caused by the effects of intermolecular forces at the interface. Since these intermolecular forces vary depending on the nature of the. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which tends to minimise. Surface tension, capillary. Surface Tension Of Liquid Depends On.
From ucscphysicsdemo.sites.ucsc.edu
Surface Tension of Liquids UCSC Physics Demonstration Room Surface Tension Of Liquid Depends On “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which tends to minimise. Surface tension is caused by the effects of intermolecular forces at the interface. Surface tension depends mainly upon the forces of attraction between the particles within the. Surface Tension Of Liquid Depends On.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Surface Tension Of Liquid Depends On The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension, capillary action, and viscosity are unique properties of liquids that depend on the nature of intermolecular interactions. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Since these intermolecular forces vary depending on the nature of the.. Surface Tension Of Liquid Depends On.
From www.slideserve.com
PPT LIQUID SURFACES PowerPoint Presentation, free download ID4456243 Surface Tension Of Liquid Depends On Surface tension is the energy required to increase. Surface tension depends on the nature of the liquid, the surrounding. The molecules on the surface of a liquid are attracted by their neighbors from the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension, capillary action, and viscosity. Surface Tension Of Liquid Depends On.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free Surface Tension Of Liquid Depends On Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with it. Surface tension depends on the nature of the liquid, the surrounding. “surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the. Surface Tension Of Liquid Depends On.