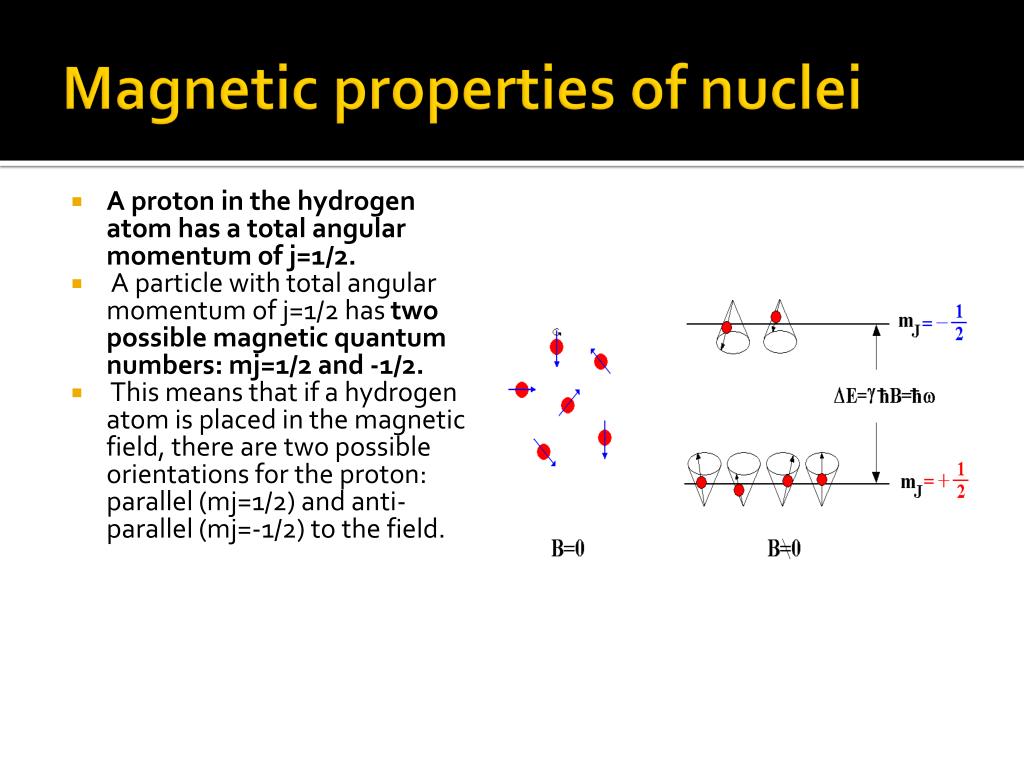
Magnetic Nuclei Examples . Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. N are particularly stable, e.g. Doubly magic nuclei are especially stable. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. Binding energy per nucleon is large for magic numbers. Fortunately for chemists, several common nuclei, including hydrogen (1 h), the 13 c isotope of carbon, the 19 f isotope of fluorine,.
from www.slideserve.com
Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. N are particularly stable, e.g. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. Binding energy per nucleon is large for magic numbers. A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. Doubly magic nuclei are especially stable. Fortunately for chemists, several common nuclei, including hydrogen (1 h), the 13 c isotope of carbon, the 19 f isotope of fluorine,. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field.
PPT The Basics of Resonance Imaging (MRI) PowerPoint
Magnetic Nuclei Examples The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. Doubly magic nuclei are especially stable. Binding energy per nucleon is large for magic numbers. Fortunately for chemists, several common nuclei, including hydrogen (1 h), the 13 c isotope of carbon, the 19 f isotope of fluorine,. A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. N are particularly stable, e.g.
From www.researchgate.net
Calculated Values of Moments of Mirror Nuclei Pairs with N Magnetic Nuclei Examples A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. Fortunately for chemists, several common nuclei, including hydrogen (1 h),. Magnetic Nuclei Examples.
From www.slideserve.com
PPT NMR Spectroscopy PowerPoint Presentation, free download ID9552301 Magnetic Nuclei Examples Doubly magic nuclei are especially stable. A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium. Magnetic Nuclei Examples.
From www.slideserve.com
PPT The Basics of Resonance Imaging (MRI) PowerPoint Magnetic Nuclei Examples N are particularly stable, e.g. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. Binding energy per nucleon is large. Magnetic Nuclei Examples.
From www.slideserve.com
PPT N uclear M R esonance Spectroscopy PowerPoint Magnetic Nuclei Examples N are particularly stable, e.g. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei. Magnetic Nuclei Examples.
From www.electricity-magnetism.org
What is a field? Magnetic Nuclei Examples A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. Binding energy per nucleon is large for magic numbers. Fortunately for. Magnetic Nuclei Examples.
From www.researchgate.net
3 Schematic describing the fundamental operating principle of nuclear Magnetic Nuclei Examples Binding energy per nucleon is large for magic numbers. Fortunately for chemists, several common nuclei, including hydrogen (1 h), the 13 c isotope of carbon, the 19 f isotope of fluorine,. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. Doubly magic nuclei are especially stable. The diagram on the left below. Magnetic Nuclei Examples.
From material-properties.org
What is Stable Nuclei Unstable Nuclei Definition Magnetic Nuclei Examples Binding energy per nucleon is large for magic numbers. Doubly magic nuclei are especially stable. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. Fortunately for chemists, several common nuclei, including hydrogen (1 h), the 13 c isotope of carbon, the. Magnetic Nuclei Examples.
From www.slideserve.com
PPT The Basics of Resonance Imaging (MRI) PowerPoint Magnetic Nuclei Examples N are particularly stable, e.g. Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei. Magnetic Nuclei Examples.
From www.slideserve.com
PPT The Basics of Resonance Imaging (MRI) PowerPoint Magnetic Nuclei Examples Binding energy per nucleon is large for magic numbers. Fortunately for chemists, several common nuclei, including hydrogen (1 h), the 13 c isotope of carbon, the 19 f isotope of fluorine,. A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n =. Magnetic Nuclei Examples.
From www.slideserve.com
PPT The Basics of Resonance Imaging (MRI) PowerPoint Magnetic Nuclei Examples Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. Binding energy per nucleon is large for magic numbers. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. Fortunately for chemists, several common nuclei, including hydrogen (1 h), the 13 c isotope of carbon,. Magnetic Nuclei Examples.
From www.slideserve.com
PPT The Basics of Resonance Imaging (MRI) PowerPoint Magnetic Nuclei Examples A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. Doubly magic nuclei are especially stable. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium. Magnetic Nuclei Examples.
From www.eclipsemagnetics.com
A Quick Guide to Metals & Metals Magnetic Nuclei Examples Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. N are particularly stable, e.g. Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei. Magnetic Nuclei Examples.
From slidetodoc.com
The Nuclei of Many Elements Are A Magnetic Nuclei Examples Binding energy per nucleon is large for magic numbers. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. Doubly magic nuclei are especially. Magnetic Nuclei Examples.
From eduinput.com
10 Examples of Nuclear Resonance Magnetic Nuclei Examples Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. N are particularly stable, e.g. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei. Magnetic Nuclei Examples.
From chem.libretexts.org
1.3 Properties of Nuclei. Nuclear Spin Chemistry LibreTexts Magnetic Nuclei Examples Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. N are particularly stable, e.g. Fortunately for chemists, several common nuclei, including hydrogen (1 h),. Magnetic Nuclei Examples.
From www.researchgate.net
SPM images of Co nuclei grown at À700 mV. (A) Topographic image; (B Magnetic Nuclei Examples A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. Doubly magic nuclei are especially stable. Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. Nuclei of a given. Magnetic Nuclei Examples.
From www.slideserve.com
PPT Nuclei With Spin Align in Fields PowerPoint Presentation Magnetic Nuclei Examples Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful. Magnetic Nuclei Examples.
From chembam.com
Nuclear Resonance Magnetic Nuclei Examples Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. Doubly magic nuclei are especially stable. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. Binding energy per nucleon is large for magic. Magnetic Nuclei Examples.
From en.ppt-online.org
Nuclear resonance spectroscopy online presentation Magnetic Nuclei Examples Fortunately for chemists, several common nuclei, including hydrogen (1 h), the 13 c isotope of carbon, the 19 f isotope of fluorine,. Binding energy per nucleon is large for magic numbers. Doubly magic nuclei are especially stable. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. N are particularly stable, e.g. The. Magnetic Nuclei Examples.
From present5.com
Introduction to Nuclear Resonance—NMR Principle of NMR Magnetic Nuclei Examples Doubly magic nuclei are especially stable. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. Nuclear magnetic resonance spectroscopy (nmr) is a widely used. Magnetic Nuclei Examples.
From en.ppt-online.org
Nuclear resonance spectroscopy online presentation Magnetic Nuclei Examples Binding energy per nucleon is large for magic numbers. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. Doubly magic nuclei are especially stable.. Magnetic Nuclei Examples.
From www.slideserve.com
PPT NMR Spectroscopy PowerPoint Presentation, free download ID4447029 Magnetic Nuclei Examples A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. Binding energy per nucleon is large for magic numbers. Fortunately for. Magnetic Nuclei Examples.
From www.youtube.com
Theory of nuclear resonance properties of nuclei Magnetic Nuclei Examples Binding energy per nucleon is large for magic numbers. A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2. Magnetic Nuclei Examples.
From www.slideserve.com
PPT NMR Spectroscopy PowerPoint Presentation, free download ID4447029 Magnetic Nuclei Examples The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. N are particularly stable, e.g. A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet. Magnetic Nuclei Examples.
From www.slideserve.com
PPT Spin Properties of Nuclei PowerPoint Presentation, free download Magnetic Nuclei Examples The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =.. Magnetic Nuclei Examples.
From www.slideserve.com
PPT Basic MRI I PowerPoint Presentation, free download ID361431 Magnetic Nuclei Examples Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. Nuclei of a given type, will resonate at different energies depending on their chemical. Magnetic Nuclei Examples.
From chem.libretexts.org
12.1 Theory of Nuclear Resonance (NMR) Chemistry LibreTexts Magnetic Nuclei Examples Binding energy per nucleon is large for magic numbers. N are particularly stable, e.g. Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. Fortunately for chemists, several common nuclei, including hydrogen (1 h), the 13 c isotope of carbon, the 19 f isotope of fluorine,. Nuclei of a given type, will. Magnetic Nuclei Examples.
From www.slideserve.com
PPT NUCLEAR RESONANCE PowerPoint Presentation, free download Magnetic Nuclei Examples Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. Fortunately for chemists, several common nuclei, including hydrogen (1 h), the 13 c isotope of carbon, the 19 f isotope of fluorine,. Binding energy per nucleon is large for magic numbers. Doubly magic nuclei are especially stable. The diagram on the left. Magnetic Nuclei Examples.
From chem.libretexts.org
NMR Theory Chemistry LibreTexts Magnetic Nuclei Examples Binding energy per nucleon is large for magic numbers. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. Nuclei of a given type,. Magnetic Nuclei Examples.
From mri-q.com
Questions and Answers in MRI Magnetic Nuclei Examples A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. Binding energy per nucleon is large for magic numbers. The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2. Magnetic Nuclei Examples.
From www.slideserve.com
PPT NMR arises from the fact that certain atomic nuclei have a Magnetic Nuclei Examples The diagram on the left below illustrates the macroscopic magnetization of a sample containing large numbers of spin 1/2 nuclei at equilibrium in a strong external magnetic field. Fortunately for chemists, several common nuclei, including hydrogen (1 h), the 13 c isotope of carbon, the 19 f isotope of fluorine,. Nuclear magnetic resonance spectroscopy (nmr) is a widely used and. Magnetic Nuclei Examples.
From www.slideserve.com
PPT Resonance Imaging PowerPoint Presentation, free download Magnetic Nuclei Examples Nuclei of a given type, will resonate at different energies depending on their chemical and electronic environment. Nuclear magnetic resonance spectroscopy (nmr) is a widely used and powerful method that takes advantage of the magnetic. Doubly magic nuclei are especially stable. Binding energy per nucleon is large for magic numbers. Fortunately for chemists, several common nuclei, including hydrogen (1 h),. Magnetic Nuclei Examples.
From www.wonkeedonkeetools.co.uk
How does a work? Wonkee Donkee Tools Magnetic Nuclei Examples Binding energy per nucleon is large for magic numbers. Fortunately for chemists, several common nuclei, including hydrogen (1 h), the 13 c isotope of carbon, the 19 f isotope of fluorine,. A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n =. Magnetic Nuclei Examples.
From alevelphysics.co.uk
Stable and Unstable Nuclei A Level Physics Revision Notes Magnetic Nuclei Examples Fortunately for chemists, several common nuclei, including hydrogen (1 h), the 13 c isotope of carbon, the 19 f isotope of fluorine,. A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. Nuclei of a given type, will. Magnetic Nuclei Examples.
From www.slideserve.com
PPT Nuclear Resonance I PowerPoint Presentation, free Magnetic Nuclei Examples N are particularly stable, e.g. A spinning charged particle possesses a characteristic magnetic moment μ and can be described as a magnetic dipole creating a magnetic field similar to a bar magnet (n = north, s =. Binding energy per nucleon is large for magic numbers. Doubly magic nuclei are especially stable. The diagram on the left below illustrates the. Magnetic Nuclei Examples.