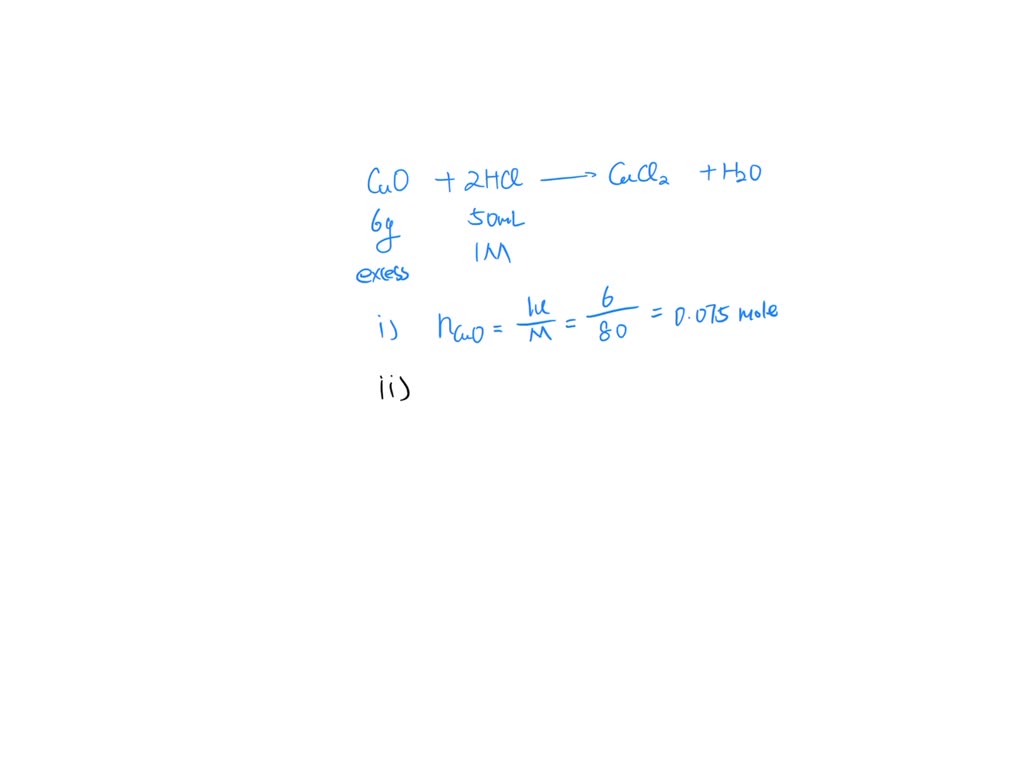Copper Oxide With Hcl . cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. It shows the reactants (substances that start a reaction) and products. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper (ii) oxide [cuo] and. a chemical equation represents a chemical reaction. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. there are three main steps for writing the net ionic equation for cuo +. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. this video demonstrates the action of acids on metal oxides.
from www.numerade.com
activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. It shows the reactants (substances that start a reaction) and products. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper (ii) oxide [cuo] and. this video demonstrates the action of acids on metal oxides. cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. there are three main steps for writing the net ionic equation for cuo +. a chemical equation represents a chemical reaction.
SOLVED Copper (II) oxide reacts with dilute hydrochloric acid. CuO(s
Copper Oxide With Hcl a chemical equation represents a chemical reaction. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. there are three main steps for writing the net ionic equation for cuo +. this video demonstrates the action of acids on metal oxides. cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. a chemical equation represents a chemical reaction. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper (ii) oxide [cuo] and. It shows the reactants (substances that start a reaction) and products. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding.
From www.youtube.com
41 Cu + HCl Copper + Hydrochloric acid💚 YouTube Copper Oxide With Hcl copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. this video demonstrates the action of acids on metal oxides. It shows the reactants (substances that start a reaction) and products. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between. Copper Oxide With Hcl.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Oxide With Hcl this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. this video demonstrates the action of acids on metal oxides. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. a chemical equation represents a chemical reaction. there. Copper Oxide With Hcl.
From askfilo.com
Recall from Chapter 2, how copper oxide reacts with hydrochloric acid. We.. Copper Oxide With Hcl there are three main steps for writing the net ionic equation for cuo +. a chemical equation represents a chemical reaction. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. this video demonstrates the action of acids on metal oxides. activity 2.7 from chapter. Copper Oxide With Hcl.
From www.numerade.com
SOLVED Write a balanced chemical reaction for the dissolution of your Copper Oxide With Hcl activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. a chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products. this video demonstrates the action of acids on metal oxides. this reaction is a type of double replacement reaction called a. Copper Oxide With Hcl.
From www.youtube.com
CuO+HCl=CuCl2+H2O Balanced EquationCopper oxide+Hydrochloric acid Copper Oxide With Hcl copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. there are three main steps for writing the net ionic equation for cuo +. this video demonstrates the action. Copper Oxide With Hcl.
From www.sciencephoto.com
Copper (II) oxide reacts with hydrochloric acid Stock Image C036 Copper Oxide With Hcl copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. this video demonstrates the action of acids on metal oxides. cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. It shows the reactants. Copper Oxide With Hcl.
From www.youtube.com
How to Balance CuO + HCl = CuCl2 + H2O YouTube Copper Oxide With Hcl this video demonstrates the action of acids on metal oxides. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. It shows the reactants (substances that start. Copper Oxide With Hcl.
From www.youtube.com
Hydrochloric Acid Reaction HCL and mixed metal copper oxides YouTube Copper Oxide With Hcl a chemical equation represents a chemical reaction. this video demonstrates the action of acids on metal oxides. there are three main steps for writing the net ionic equation for cuo +. cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. activity. Copper Oxide With Hcl.
From www.youtube.com
Reaction of copper oxide with Hcl YouTube Copper Oxide With Hcl It shows the reactants (substances that start a reaction) and products. cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. a chemical equation represents a chemical reaction. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid. Copper Oxide With Hcl.
From www.youtube.com
How to Write the Net Ionic Equation for CuO + HCl = CuCl2 + H2O YouTube Copper Oxide With Hcl activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid.. Copper Oxide With Hcl.
From www.numerade.com
SOLVED 6. Copper(II) oxide reacts with hydrochloric acid to produce Copper Oxide With Hcl It shows the reactants (substances that start a reaction) and products. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. cuo + hcl = cucl2 + h2o is a double displacement (metathesis). Copper Oxide With Hcl.
From www.sciencephoto.com
Copper in hydrochloric acid Stock Image A500/0755 Science Photo Copper Oxide With Hcl there are three main steps for writing the net ionic equation for cuo +. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. this video demonstrates the action of acids on metal oxides. this reaction is a type of double replacement reaction called a neutralization reaction, in which. Copper Oxide With Hcl.
From www.numerade.com
SOLVED Copper (II) oxide reacts with dilute hydrochloric acid. CuO(s Copper Oxide With Hcl cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. there are three main steps for writing the net ionic equation for cuo +. It shows the. Copper Oxide With Hcl.
From www.youtube.com
Making copper hydroxide YouTube Copper Oxide With Hcl cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper (ii) oxide [cuo] and. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. there are three main steps for writing the net ionic equation for cuo +. . Copper Oxide With Hcl.
From www.youtube.com
Copper Cycle 05 02 copper oxide +HCl 02 YouTube Copper Oxide With Hcl cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper (ii) oxide [cuo] and. this video demonstrates the action of acids on metal oxides. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. It shows the reactants (substances that start a. Copper Oxide With Hcl.
From www.youtube.com
Copper & Hydrochloric Acid Ligand Substitution YouTube Copper Oxide With Hcl this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. this video demonstrates the action of acids on metal oxides. a chemical equation represents a chemical reaction. It shows. Copper Oxide With Hcl.
From www.youtube.com
Reaction of copper oxide with dilute HClscience activity class 10 Copper Oxide With Hcl this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper (ii) oxide [cuo] and. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give. Copper Oxide With Hcl.
From www.youtube.com
81 CuO + HCl Copper oxide + Hydrochloric acid💚 YouTube Copper Oxide With Hcl there are three main steps for writing the net ionic equation for cuo +. a chemical equation represents a chemical reaction. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. this video demonstrates the action of acids on metal oxides. cu2o + hcl =. Copper Oxide With Hcl.
From www.toppr.com
On adding dilute hydrochloric acid to copper oxide powder, the solution Copper Oxide With Hcl It shows the reactants (substances that start a reaction) and products. a chemical equation represents a chemical reaction. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. this video demonstrates the action of acids on metal oxides. cuo + hcl = cucl2 + h2o is. Copper Oxide With Hcl.
From www.sciencephoto.com
Two copper oxide reactions Stock Image A500/0465 Science Photo Library Copper Oxide With Hcl cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper (ii) oxide [cuo] and. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. this video demonstrates the action of acids on metal oxides. cu2o + hcl = cucl + h2o. Copper Oxide With Hcl.
From www.gkseries.com
When copper oxide and dilute hydrochloric acid react, colour changes to Copper Oxide With Hcl this video demonstrates the action of acids on metal oxides. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper (ii) oxide [cuo] and. a chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products. activity 2.7 from chapter 2 of. Copper Oxide With Hcl.
From www.youtube.com
CuO + HCl YouTube Copper Oxide With Hcl activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. there are three main steps for writing the net ionic equation for cuo +. this video demonstrates the action of acids on metal oxides. cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one. Copper Oxide With Hcl.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Oxide With Hcl activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. a chemical equation represents a chemical reaction. cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. this reaction is a type of double replacement reaction. Copper Oxide With Hcl.
From www.youtube.com
Reaction of Metallic oxides YouTube Copper Oxide With Hcl It shows the reactants (substances that start a reaction) and products. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper (ii) oxide [cuo] and. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. activity 2.7 from chapter. Copper Oxide With Hcl.
From www.researchgate.net
Synthesis of copper oxide nanoparticles (a) copper chloride (d) copper Copper Oxide With Hcl this video demonstrates the action of acids on metal oxides. this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. a chemical equation represents a chemical reaction. there are three main steps for writing the net ionic equation for cuo +. cu2o + hcl = cucl + h2o. Copper Oxide With Hcl.
From brainly.com
The diagram below shows a stage in the preparation of copper chloride Copper Oxide With Hcl this video demonstrates the action of acids on metal oxides. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. there are three main steps for writing the net ionic equation for cuo +. a chemical equation represents a chemical reaction. cu2o + hcl =. Copper Oxide With Hcl.
From www.youtube.com
Type of Reaction for Cu + HCl (Copper + Hydrochloric acid) YouTube Copper Oxide With Hcl activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. there are three main steps for writing the net ionic equation for cuo +. a chemical equation represents a. Copper Oxide With Hcl.
From www.youtube.com
On adding dilute hydrochloric acid to copper oxide powder, the solu Copper Oxide With Hcl this video demonstrates the action of acids on metal oxides. a chemical equation represents a chemical reaction. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper (ii) oxide [cuo] and. It shows the reactants (substances that start a reaction) and products. copper (ii) oxide reacts with mineral. Copper Oxide With Hcl.
From cexoxhzj.blob.core.windows.net
Copper Oxide And Carbon Reaction at Tracey Miller blog Copper Oxide With Hcl cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper (ii) oxide [cuo] and. It shows the reactants (substances that start a reaction) and products. cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. this. Copper Oxide With Hcl.
From www.youtube.com
Copper Cycle 05 03 copper oxide +HCl 03 YouTube Copper Oxide With Hcl It shows the reactants (substances that start a reaction) and products. this video demonstrates the action of acids on metal oxides. there are three main steps for writing the net ionic equation for cuo +. a chemical equation represents a chemical reaction. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where. Copper Oxide With Hcl.
From www.toppr.com
Take a small amount of copper oxide in a beaker and add dilute Copper Oxide With Hcl there are three main steps for writing the net ionic equation for cuo +. cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding.. Copper Oxide With Hcl.
From www.youtube.com
Cu + H2SO4 (Copper + Sulfuric acid) YouTube Copper Oxide With Hcl a chemical equation represents a chemical reaction. cuo + hcl = cucl2 + h2o is a double displacement (metathesis) reaction where one mole of copper (ii) oxide [cuo] and. activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. copper (ii) oxide reacts with mineral acids such as hydrochloric. Copper Oxide With Hcl.
From www.youtube.com
Copper Cycle 05 01 copper oxide +HCl 01 YouTube Copper Oxide With Hcl activity 2.7 from chapter 2 of the ncert class 10 science textbook demonstrates the reaction between a. cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. there are three main steps for writing the net ionic equation for cuo +. a chemical. Copper Oxide With Hcl.
From brainly.in
On adding dilute hcl to Copper oxide powder the solution formed blue Copper Oxide With Hcl this reaction is a type of double replacement reaction called a neutralization reaction, in which the solid. there are three main steps for writing the net ionic equation for cuo +. copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. this video demonstrates the action. Copper Oxide With Hcl.
From www.youtube.com
Reaction of Copper Oxide With Hydrochloric Acid YouTube Copper Oxide With Hcl copper (ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding. this video demonstrates the action of acids on metal oxides. cu2o + hcl = cucl + h2o is a double displacement (metathesis) reaction where one mole of copper(i) oxide [cu 2 o] and. It shows the reactants. Copper Oxide With Hcl.