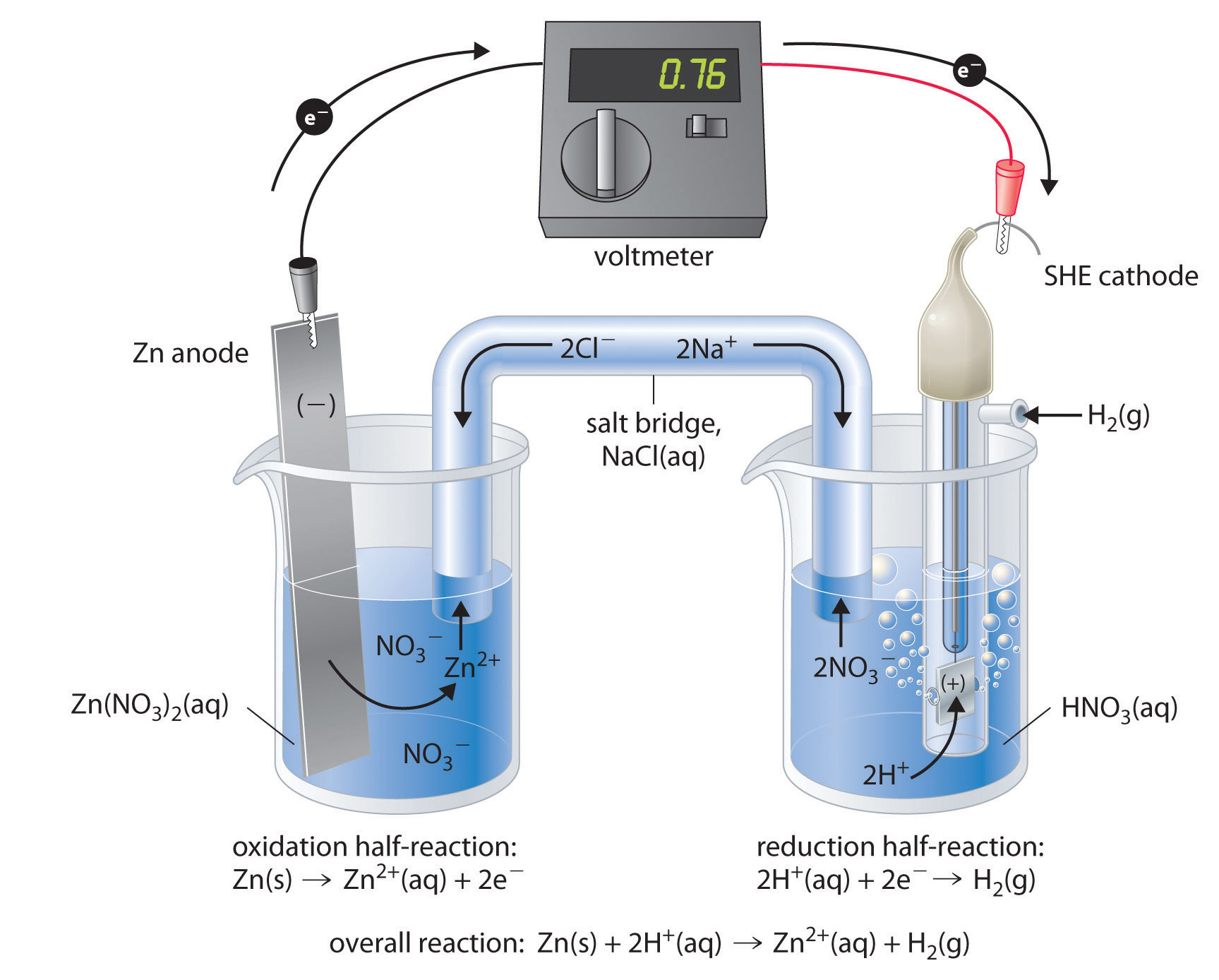
Fuel Cells In Electrochemistry . fuel cells are efficient energy converters, based on electrochemical principles. Fuel cells are similar to batteries. A fuel cell is a device that converts chemical energy into electrical energy. They convert the chemical energy (heating. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available.
from saylordotorg.github.io
a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. They convert the chemical energy (heating. A fuel cell is a device that converts chemical energy into electrical energy. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. fuel cells are efficient energy converters, based on electrochemical principles. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. Fuel cells are similar to batteries. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time.
Electrochemistry
Fuel Cells In Electrochemistry A fuel cell is a device that converts chemical energy into electrical energy. A fuel cell is a device that converts chemical energy into electrical energy. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to. Fuel cells are similar to batteries. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cells are efficient energy converters, based on electrochemical principles. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. They convert the chemical energy (heating. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time.
From www.alamy.com
Block diagram of fuel cell. Schematic diagram of hydrogen fuel cell Fuel Cells In Electrochemistry fuel cells are efficient energy converters, based on electrochemical principles. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. Fuel cells are similar to batteries. A fuel cell is a device that converts chemical energy into electrical energy. fuel cell, any of a class of devices that convert. Fuel Cells In Electrochemistry.
From www.zastita-materijala.org
Bacillus amyloliquefaciens strain NSB4 bacteria for treating wastewater Fuel Cells In Electrochemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. a battery is a contained unit that produces electricity, whereas a fuel. Fuel Cells In Electrochemistry.
From iasgoogle.com
IAS Google Cracking IAS Academy Fuel Cells In Electrochemistry fuel cells are efficient energy converters, based on electrochemical principles. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. A fuel cell resembles a. Fuel Cells In Electrochemistry.
From jphechsi.blogspot.com
electrochemistry Hydrogen fuel cell why do the H+ ions move through Fuel Cells In Electrochemistry A fuel cell is a device that converts chemical energy into electrical energy. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. fuel cells are efficient energy converters, based on electrochemical principles. a fuel cell like this will continue to operate and produce electrical energy. Fuel Cells In Electrochemistry.
From www.semanticscholar.org
Figure 3 from Electrolytic CO2 Reduction in a Flow Cell. Semantic Scholar Fuel Cells In Electrochemistry Fuel cells are similar to batteries. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cells are efficient energy converters, based on electrochemical. Fuel Cells In Electrochemistry.
From saylordotorg.github.io
Electrochemistry Fuel Cells In Electrochemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells are similar to batteries. covers all aspects of fuel cell fundamentals, including. Fuel Cells In Electrochemistry.
From saylordotorg.github.io
Electrochemistry Fuel Cells In Electrochemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to. A fuel cell is a device that converts. Fuel Cells In Electrochemistry.
From mavink.com
Diagram Of Electrochemical Cell Fuel Cells In Electrochemistry fuel cells are efficient energy converters, based on electrochemical principles. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. A fuel cell is a device that converts. Fuel Cells In Electrochemistry.
From pubs.rsc.org
Making electrochemistry easily accessible to the synthetic chemist Fuel Cells In Electrochemistry A fuel cell is a device that converts chemical energy into electrical energy. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are similar to. Fuel Cells In Electrochemistry.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Fuel Cells In Electrochemistry A fuel cell is a device that converts chemical energy into electrical energy. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. They convert the chemical energy (heating. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into. Fuel Cells In Electrochemistry.
From ubicaciondepersonas.cdmx.gob.mx
Fuel Cell Vector Stock Vector Colourbox ubicaciondepersonas.cdmx.gob.mx Fuel Cells In Electrochemistry A fuel cell is a device that converts chemical energy into electrical energy. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant. Fuel Cells In Electrochemistry.
From saylordotorg.github.io
Electrolysis Fuel Cells In Electrochemistry A fuel cell is a device that converts chemical energy into electrical energy. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. fuel cells are efficient energy converters, based on electrochemical principles. They convert the chemical energy (heating. Fuel cells are similar to batteries. A. Fuel Cells In Electrochemistry.
From chem.libretexts.org
20.7 Batteries and Fuel Cells Chemistry LibreTexts Fuel Cells In Electrochemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is a device that converts chemical energy into electrical energy.. Fuel Cells In Electrochemistry.
From mrselliott.wordpress.com
Electrochemistry, featuring electrolysis and fuel cells. mrs elliott Fuel Cells In Electrochemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell is a device that converts chemical energy into electrical energy. They convert the chemical energy (heating. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief.. Fuel Cells In Electrochemistry.
From beamonics.se
Comparing Gas Analysis TDLAS, NDIR and Electrochemical Cells Beamonics Fuel Cells In Electrochemistry a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells are similar to batteries. fuel cells are efficient energy converters, based on electrochemical principles. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell. Fuel Cells In Electrochemistry.
From www.preprints.org
Educational Model of Electric Potential, Electrochemical Reactions, and Fuel Cells In Electrochemistry fuel cells are efficient energy converters, based on electrochemical principles. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to. a fuel cell like this will continue to operate and produce electrical energy as long as a supply. Fuel Cells In Electrochemistry.
From www.chemcz.com
محاضرات الكيمياء الكهربية (الفرق الثالثة جميع الشعب) CHEM CLUB Fuel Cells In Electrochemistry They convert the chemical energy (heating. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. fuel cells are efficient energy converters, based on electrochemical principles. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. . Fuel Cells In Electrochemistry.
From www.mdpi.com
Catalysts Free FullText Fundamentals of Gas Diffusion Electrodes Fuel Cells In Electrochemistry A fuel cell is a device that converts chemical energy into electrical energy. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to. They convert the chemical energy (heating. A fuel cell resembles a battery in many respects, but it. Fuel Cells In Electrochemistry.
From www.mathworks.com
Fuel Cell Model MATLAB & Simulink Fuel Cells In Electrochemistry covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. fuel cells are efficient energy converters, based on electrochemical principles. They convert the chemical energy (heating. fuel. Fuel Cells In Electrochemistry.
From www.researchgate.net
e Schematic view of a typical hydrogen PEM fuel cell and its components Fuel Cells In Electrochemistry A fuel cell is a device that converts chemical energy into electrical energy. They convert the chemical energy (heating. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to. a fuel cell like this will continue to operate and. Fuel Cells In Electrochemistry.
From library.achievingthedream.org
Phases and Classification of Matter Introductory Chemistry Lecture Fuel Cells In Electrochemistry They convert the chemical energy (heating. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. fuel cells are efficient energy converters, based on electrochemical principles. Fuel cells are similar to batteries. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts,. Fuel Cells In Electrochemistry.
From www.hotzxgirl.com
Pem Fuel Cell Hot Sex Picture Fuel Cells In Electrochemistry a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. They convert the chemical energy (heating. A. Fuel Cells In Electrochemistry.
From www.pinterest.co.kr
Galvanic Cell and Electrolytic Cell Electrochemical Cells Fuel Cells In Electrochemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is a device that converts chemical energy into electrical energy.. Fuel Cells In Electrochemistry.
From exywogfyd.blob.core.windows.net
General Chemistry 2 Electrochemistry at Precious Werner blog Fuel Cells In Electrochemistry A fuel cell is a device that converts chemical energy into electrical energy. Fuel cells are similar to batteries. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cells are efficient energy converters, based on electrochemical principles. fuel cell, any of. Fuel Cells In Electrochemistry.
From circuitlisthoughed88.z13.web.core.windows.net
Cell Diagram Voltaic Cell Fuel Cells In Electrochemistry They convert the chemical energy (heating. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel. Fuel Cells In Electrochemistry.
From saylordotorg.github.io
Standard Potentials Fuel Cells In Electrochemistry a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell resembles a. Fuel Cells In Electrochemistry.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Fuel Cells In Electrochemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are similar to batteries. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. A fuel cell is a device that converts chemical energy into electrical. Fuel Cells In Electrochemistry.
From 2012books.lardbucket.org
Electrochemistry Fuel Cells In Electrochemistry a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. covers all aspects of fuel cell fundamentals, including their basic thermodynamics,. Fuel Cells In Electrochemistry.
From www.plugpower.com
Fuel Cells What They Are and How They Work Plug Power Fuel Cells In Electrochemistry fuel cells are efficient energy converters, based on electrochemical principles. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel. Fuel Cells In Electrochemistry.
From www.pinterest.com
Image result for explain about fuel cells Electrochemistry, Fuel cell Fuel Cells In Electrochemistry a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. A fuel cell is a device that converts chemical energy into electrical energy. a battery. Fuel Cells In Electrochemistry.
From giolynujr.blob.core.windows.net
Chemical Reaction Within Hydrogen Fuel Cell at Wilkerson blog Fuel Cells In Electrochemistry a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to. fuel cells are efficient energy converters, based on electrochemical principles. A fuel cell is a device that converts chemical energy into electrical energy. Fuel cells are similar to batteries.. Fuel Cells In Electrochemistry.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Fuel Cells In Electrochemistry fuel cells are efficient energy converters, based on electrochemical principles. covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. a fuel cell like this will continue. Fuel Cells In Electrochemistry.
From www.youtube.com
Fuel Cell (0601) Thermodynamics Electrochem Reaction YouTube Fuel Cells In Electrochemistry covers all aspects of fuel cell fundamentals, including their basic thermodynamics, electrochemistry, electrocatalysts, and materials, plus a brief. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell resembles a battery in many respects, but it can supply electrical energy over. Fuel Cells In Electrochemistry.
From www.desertcart.sg
Buy Modeling, Analysis, and Optimal Control of Fuel Cell Fuel Cells In Electrochemistry They convert the chemical energy (heating. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. a battery is a contained unit that produces electricity, whereas a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants. Fuel Cells In Electrochemistry.
From www.gecos.polimi.it
Hydrogen, Fuel Cells and Electrochemical Energy Systems GECOS page Fuel Cells In Electrochemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell is a device that converts chemical energy into electrical energy. fuel cells are efficient energy converters, based on electrochemical principles. A fuel cell resembles a battery in many respects, but it can supply. Fuel Cells In Electrochemistry.