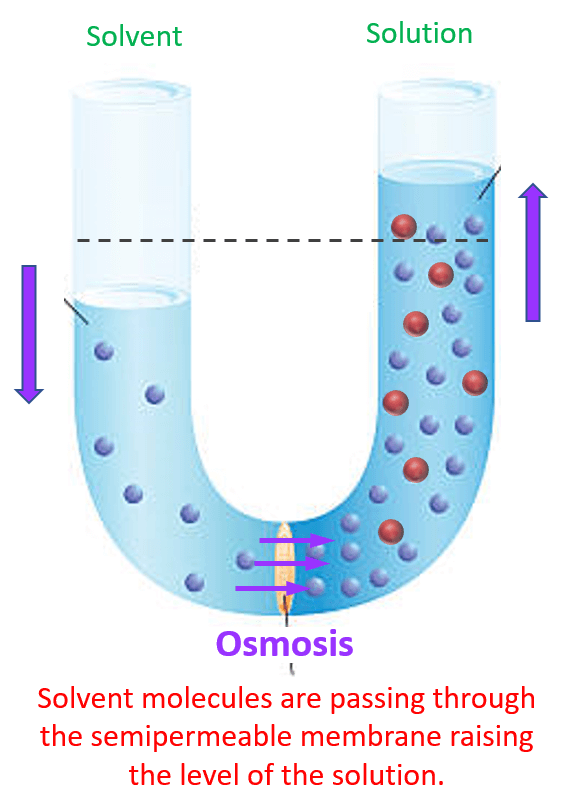
What Solution Causes Osmosis . A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. finally, an isotonic solution is one that causes no change in the cell. You can imagine that the solution and the cell have equal concentrations, so there. osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. If a cell has a cell wall, the wall helps maintain the cell’s water balance. when water moves into a cell by osmosis, osmotic pressure may build up inside the cell. in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure.
from general.chemistrysteps.com
osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. If a cell has a cell wall, the wall helps maintain the cell’s water balance. osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. finally, an isotonic solution is one that causes no change in the cell. You can imagine that the solution and the cell have equal concentrations, so there. when water moves into a cell by osmosis, osmotic pressure may build up inside the cell.
Osmotic Pressure Chemistry Steps
What Solution Causes Osmosis osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. finally, an isotonic solution is one that causes no change in the cell. osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. If a cell has a cell wall, the wall helps maintain the cell’s water balance. when water moves into a cell by osmosis, osmotic pressure may build up inside the cell. in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. You can imagine that the solution and the cell have equal concentrations, so there.
From kmbiology.weebly.com
Osmosis Notes Biology Mrs. What Solution Causes Osmosis when water moves into a cell by osmosis, osmotic pressure may build up inside the cell. finally, an isotonic solution is one that causes no change in the cell. in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. A cell in an isotonic solution is in equilibrium with. What Solution Causes Osmosis.
From www.priyamstudycentre.com
Osmosis Definition, Example, Osmotic Pressure What Solution Causes Osmosis in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. You can imagine that the solution and the cell have equal concentrations, so there. osmosis is defined as the spontaneous movement of solvent molecules. What Solution Causes Osmosis.
From www.vedantu.com
Osmosis is an example of active transport. True or False? Explain. What Solution Causes Osmosis osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. If a cell has a cell wall, the wall helps maintain the cell’s water balance. A cell in. What Solution Causes Osmosis.
From preparatorychemistry.com
Osmosis What Solution Causes Osmosis finally, an isotonic solution is one that causes no change in the cell. osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. You can imagine that the solution and the cell have equal concentrations, so there. If a cell has a cell wall, the. What Solution Causes Osmosis.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps What Solution Causes Osmosis finally, an isotonic solution is one that causes no change in the cell. You can imagine that the solution and the cell have equal concentrations, so there. If a cell has a cell wall, the wall helps maintain the cell’s water balance. osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute. What Solution Causes Osmosis.
From driverlayer.com
osmosis DriverLayer Search Engine What Solution Causes Osmosis in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. osmosis causes net water flow from the less concentrated to the more concentrated solution across the. What Solution Causes Osmosis.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps What Solution Causes Osmosis when water moves into a cell by osmosis, osmotic pressure may build up inside the cell. osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure.. What Solution Causes Osmosis.
From sciencestruck.com
Osmosis Vs. Diffusion How are They Different From Each Other What Solution Causes Osmosis in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. You. What Solution Causes Osmosis.
From www.youtube.com
Solving problems involving osmotic pressure YouTube What Solution Causes Osmosis osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. You can imagine that the solution and the cell have equal concentrations, so there. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. in physiology, osmosis (greek for push) is the net movement of. What Solution Causes Osmosis.
From courses.lumenlearning.com
Osmoregulation and Osmotic Balance OpenStax Biology 2e What Solution Causes Osmosis osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. when water moves into a cell by osmosis, osmotic pressure may build up inside the cell. You can imagine that the solution and the cell have equal concentrations, so there. If a cell has a. What Solution Causes Osmosis.
From www.osmosis.org
Hypernatremia Osmosis What Solution Causes Osmosis osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see. What Solution Causes Osmosis.
From sciencevivid.com
Osmosis Introduction, Types, Solution, Osmotic pressure, Factors What Solution Causes Osmosis in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. If a cell has a cell wall, the wall helps maintain the cell’s water balance. osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. You can. What Solution Causes Osmosis.
From pharmaceuticalmicrobiologi.blogspot.com
PHARMACEUTICAL MICROBIOLOGY Osmotic Pressure What Solution Causes Osmosis in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. when water moves into a cell by osmosis, osmotic pressure may build up inside the cell. If a cell has a cell wall, the. What Solution Causes Osmosis.
From www.sciencefacts.net
Osmosis Definition and How Does it Occur (with Diagram) What Solution Causes Osmosis osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. You can imagine that the solution and the cell have equal concentrations, so there. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. in physiology, osmosis (greek for. What Solution Causes Osmosis.
From www.alamy.com
Osmosis experiment, illustration Stock Photo Alamy What Solution Causes Osmosis osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. If a cell has a cell wall, the wall helps maintain the cell’s water balance. osmosis is defined as the spontaneous movement of solvent molecules. What Solution Causes Osmosis.
From netsolwater.com
Why reverse osmosis has a water rescuing or saving technology What Solution Causes Osmosis osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. when water moves into a cell by osmosis, osmotic pressure may build up inside the cell. A cell in an isotonic solution. What Solution Causes Osmosis.
From www.britannica.com
Osmosis Definition, Examples, & Facts Britannica What Solution Causes Osmosis You can imagine that the solution and the cell have equal concentrations, so there. osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. when water moves into a cell by osmosis, osmotic pressure may build up inside the cell. A cell in an isotonic solution is in equilibrium with its. What Solution Causes Osmosis.
From www.youtube.com
osmosis and osmotic pressure solutions solutions class 12 chemistry What Solution Causes Osmosis osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. finally, an isotonic solution is one that causes no change in the cell. osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. in physiology, osmosis. What Solution Causes Osmosis.
From www.differencebetween.com
Difference Between Osmosis and Plasmolysis Compare the Difference What Solution Causes Osmosis A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. You can imagine that the solution and the cell have equal concentrations, so there. osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. finally, an isotonic solution is one that causes no change in. What Solution Causes Osmosis.
From kunduz.com
Osmotic Pressure Definition, Formula, Equation, Significance, Examples What Solution Causes Osmosis A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. when water moves into a cell by osmosis, osmotic pressure may build up inside the cell. osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. You can imagine that the solution and the cell. What Solution Causes Osmosis.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps What Solution Causes Osmosis in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. finally, an isotonic solution is one. What Solution Causes Osmosis.
From www.expii.com
Osmosis (Passive Transport) — Definition & Importance Expii What Solution Causes Osmosis A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. finally, an isotonic solution is one. What Solution Causes Osmosis.
From ar.inspiredpencil.com
Osmosis And Diffusion What Solution Causes Osmosis when water moves into a cell by osmosis, osmotic pressure may build up inside the cell. You can imagine that the solution and the cell have equal concentrations, so there. osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. A cell in an isotonic. What Solution Causes Osmosis.
From biologicalmembranetransport.weebly.com
Osmosis BioEdu What Solution Causes Osmosis A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. You can imagine that the solution and the cell have equal concentrations, so there. finally, an isotonic solution is one that causes no change in the cell. in physiology, osmosis (greek for push) is the net movement of water across a semipermeable. What Solution Causes Osmosis.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID What Solution Causes Osmosis finally, an isotonic solution is one that causes no change in the cell. osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. You can imagine that the solution and the cell have equal concentrations, so there. in physiology, osmosis (greek for push) is. What Solution Causes Osmosis.
From solutionpharmacy.in
Effect of Osmotic Pressure on Red Blood Cells (RBCs) What Solution Causes Osmosis finally, an isotonic solution is one that causes no change in the cell. in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. osmosis causes net water flow from the less concentrated to. What Solution Causes Osmosis.
From fyofvnyxb.blob.core.windows.net
Science Definition Hypotonic at Grace Huie blog What Solution Causes Osmosis osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. osmosis causes net water flow from the less concentrated to the more concentrated solution across the. What Solution Causes Osmosis.
From depositphotos.com
Osmosis reverse vector illustration. Explained process with solution What Solution Causes Osmosis osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. If a cell has a cell wall, the wall helps maintain the cell’s water balance. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. when water moves into. What Solution Causes Osmosis.
From gioojosqd.blob.core.windows.net
Osmosis In Anatomy at Helen Steele blog What Solution Causes Osmosis osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. when water moves into a cell by osmosis, osmotic pressure may build up inside the cell. If a cell has a cell wall, the wall helps maintain the cell’s water balance. finally, an isotonic. What Solution Causes Osmosis.
From www.slideserve.com
PPT What is osmosis? PowerPoint Presentation, free download ID2712925 What Solution Causes Osmosis in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. If a cell has a cell wall, the wall helps maintain the cell’s water balance. finally, an isotonic solution is one that causes no change in the cell. osmosis causes net water flow from the less concentrated to the. What Solution Causes Osmosis.
From www.studocu.com
Osmosis and diffusion Name What Solution Causes Osmosis If a cell has a cell wall, the wall helps maintain the cell’s water balance. You can imagine that the solution and the cell have equal concentrations, so there. in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. A cell in an isotonic solution is in equilibrium with its surroundings,. What Solution Causes Osmosis.
From www.thenakedscientists.com
The Entropic Force; 5th force of nature. Page 1 Naked Science Forum What Solution Causes Osmosis If a cell has a cell wall, the wall helps maintain the cell’s water balance. You can imagine that the solution and the cell have equal concentrations, so there. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. when water moves into a cell by osmosis, osmotic pressure may build up inside. What Solution Causes Osmosis.
From www.slideserve.com
PPT Cellular Transport PowerPoint Presentation ID6932620 What Solution Causes Osmosis A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. finally, an isotonic solution is one that causes no change in the cell. If a cell has a cell wall, the wall helps maintain the cell’s water balance. in physiology, osmosis (greek for push) is the net movement of water across a. What Solution Causes Osmosis.
From www.carlsonstockart.com
Osmosis Carlson Stock Art What Solution Causes Osmosis in physiology, osmosis (greek for push) is the net movement of water across a semipermeable membrane (see figure. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. If a cell has a cell wall, the wall helps maintain the cell’s water balance. when water moves into a cell by osmosis, osmotic. What Solution Causes Osmosis.
From luzdesnhdavila.blogspot.com
Explain the Similarities and Differences of Diffusion and Osmosis What Solution Causes Osmosis osmosis is defined as the spontaneous movement of solvent molecules from a region of low solute concentration to a region of high solute. A cell in an isotonic solution is in equilibrium with its surroundings, meaning the solute concentrations. osmosis causes net water flow from the less concentrated to the more concentrated solution across the semipermeable. If a. What Solution Causes Osmosis.