What Is A Threshold Energy In Chemistry . activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. The difference between these two values is the activation. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. For a chemical reaction to occur, an energy threshold must be. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. the threshold energy is the energy that these molecules must have in order for a reaction to take place. In other words, the kinetic. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. This equation is valid if the energies are much less than rest mass energies of the involved particles.
from stock.adobe.com
calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. In other words, the kinetic. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. the threshold energy is the energy that these molecules must have in order for a reaction to take place. For a chemical reaction to occur, an energy threshold must be. activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. This equation is valid if the energies are much less than rest mass energies of the involved particles. The difference between these two values is the activation. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant.
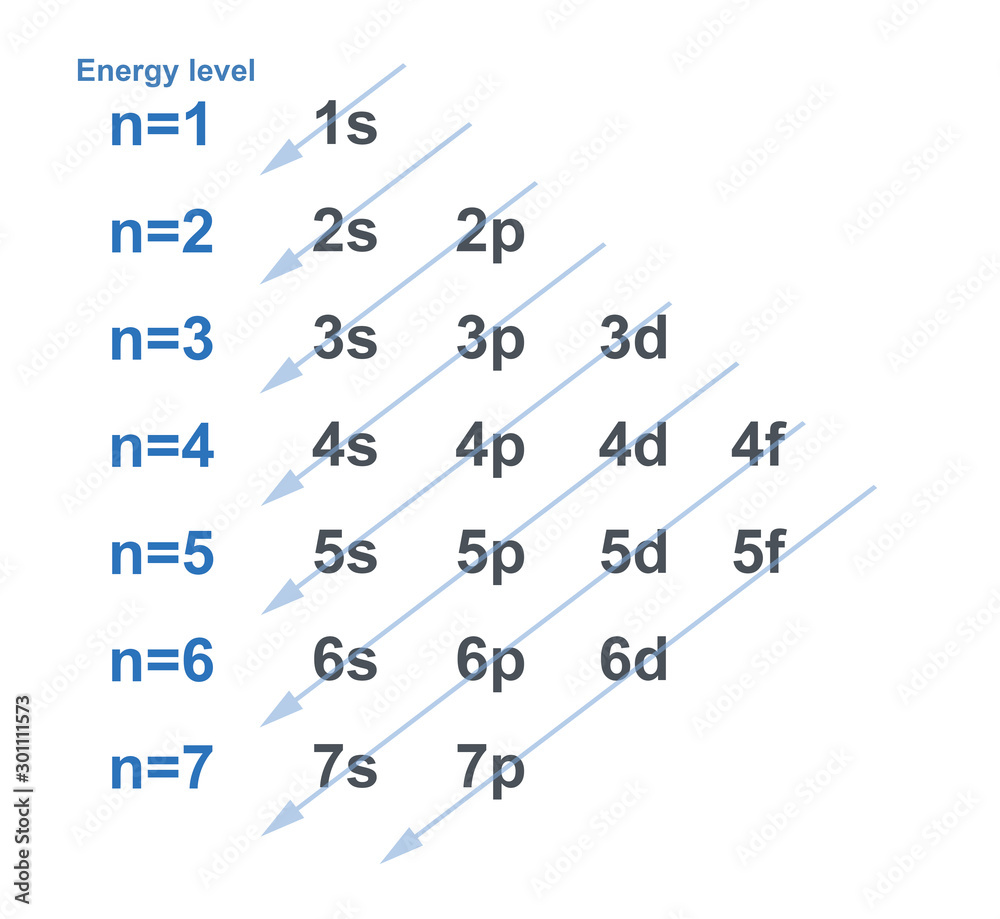
chart of electron configuration with each energy level for element in
What Is A Threshold Energy In Chemistry In other words, the kinetic. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant. activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. This equation is valid if the energies are much less than rest mass energies of the involved particles. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. The difference between these two values is the activation. In other words, the kinetic. the threshold energy is the energy that these molecules must have in order for a reaction to take place. For a chemical reaction to occur, an energy threshold must be. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur.
From kunduz.com
[ANSWERED] What is the threshold energy of reaction RP in represented What Is A Threshold Energy In Chemistry This equation is valid if the energies are much less than rest mass energies of the involved particles. In other words, the kinetic. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon. What Is A Threshold Energy In Chemistry.
From www.youtube.com
CHEM 101 Photoelectric Effect Threshold Frequency and Binding Energy What Is A Threshold Energy In Chemistry collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. the threshold energy is the energy that these molecules must have in order for a reaction to take place. The difference between these. What Is A Threshold Energy In Chemistry.
From www.periodic-table.org
What is Critical Energy Threshold Energy for Fission Definition What Is A Threshold Energy In Chemistry the threshold energy is the energy that these molecules must have in order for a reaction to take place. For a chemical reaction to occur, an energy threshold must be. The difference between these two values is the activation. In other words, the kinetic. in chemical reactions, the energy barrier corresponds to the amount of energy the particles. What Is A Threshold Energy In Chemistry.
From classnotes.org.in
Arrhenius Equation and Activation Energy Chemical Chemistry What Is A Threshold Energy In Chemistry The difference between these two values is the activation. For a chemical reaction to occur, an energy threshold must be. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. This equation is valid if the energies are much less than rest mass energies of the involved particles. . What Is A Threshold Energy In Chemistry.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image What Is A Threshold Energy In Chemistry calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. In other words, the kinetic. activation energy is primarily associated with chemical reactions, where it represents. What Is A Threshold Energy In Chemistry.
From www.youtube.com
Explain Q Value and, Threshold energy Nuclear Chemistry Physical What Is A Threshold Energy In Chemistry This equation is valid if the energies are much less than rest mass energies of the involved particles. activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The difference between these two values. What Is A Threshold Energy In Chemistry.
From byjus.com
What is threshold frequency and threshold energy? What Is A Threshold Energy In Chemistry This equation is valid if the energies are much less than rest mass energies of the involved particles. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. the threshold energy. What Is A Threshold Energy In Chemistry.
From www.purechemistry.org
Factors effecting reaction rates Temperature, catalyst Purechemistry What Is A Threshold Energy In Chemistry In other words, the kinetic. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. the threshold energy is the energy that these molecules must have in order for a reaction. What Is A Threshold Energy In Chemistry.
From byjus.com
What happens when the energy of a reaction if more than threshold What Is A Threshold Energy In Chemistry activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. the threshold. What Is A Threshold Energy In Chemistry.
From www.slideserve.com
PPT CHEM 312 Lecture 9 Nuclear Reactions PowerPoint Presentation What Is A Threshold Energy In Chemistry In other words, the kinetic. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant. For a chemical reaction to occur, an energy threshold must be. calculate. What Is A Threshold Energy In Chemistry.
From byjus.com
26. What is difference between threshold energy and activation energy? What Is A Threshold Energy In Chemistry activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. the threshold energy is the energy that these molecules must have in order for a reaction to take. What Is A Threshold Energy In Chemistry.
From userdatatactilists.z14.web.core.windows.net
Potential Energy Diagram Labeled Chemistry What Is A Threshold Energy In Chemistry in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. This equation is valid if the energies are much less than rest mass energies of the involved particles. For a chemical reaction to occur, an energy threshold must be. threshold energy is the minimum kinetic energy the. What Is A Threshold Energy In Chemistry.
From www.youtube.com
Concept of Activation Energy, Threshold Energy Chemistry(12th What Is A Threshold Energy In Chemistry collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. The difference between these two values is the activation. For a chemical reaction to occur, an energy threshold must be. activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. threshold energy is. What Is A Threshold Energy In Chemistry.
From www.sliderbase.com
Energy Levels, Sublevels, Electrons What Is A Threshold Energy In Chemistry The difference between these two values is the activation. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. the threshold energy is the energy that these molecules. What Is A Threshold Energy In Chemistry.
From www.vrogue.co
What The Significance Of “er” In The Diagram A Av vrogue.co What Is A Threshold Energy In Chemistry The difference between these two values is the activation. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. For a chemical reaction to occur, an energy threshold must. What Is A Threshold Energy In Chemistry.
From stock.adobe.com
chart of electron configuration with each energy level for element in What Is A Threshold Energy In Chemistry calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. The difference between these two values is the activation. the threshold energy is the energy that these molecules must have in order for a reaction to take place. This equation is valid if the energies are much less. What Is A Threshold Energy In Chemistry.
From exogobhlj.blob.core.windows.net
Threshold Energy For Proton at Irene Browning blog What Is A Threshold Energy In Chemistry This equation is valid if the energies are much less than rest mass energies of the involved particles. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. In. What Is A Threshold Energy In Chemistry.
From exogobhlj.blob.core.windows.net
Threshold Energy For Proton at Irene Browning blog What Is A Threshold Energy In Chemistry in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. the threshold energy is the energy that these molecules must have in order for a reaction to take. What Is A Threshold Energy In Chemistry.
From www.youtube.com
CHEMISTRY 101 Photoelectric Effect, Threshold Frequency YouTube What Is A Threshold Energy In Chemistry This equation is valid if the energies are much less than rest mass energies of the involved particles. activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. For a chemical reaction to occur, an energy threshold must be. the threshold energy is the energy that these molecules must have. What Is A Threshold Energy In Chemistry.
From www.slideserve.com
PPT Reaction Mechanisms PowerPoint Presentation, free download ID What Is A Threshold Energy In Chemistry In other words, the kinetic. For a chemical reaction to occur, an energy threshold must be. This equation is valid if the energies are much less than rest mass energies of the involved particles. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. threshold energy is. What Is A Threshold Energy In Chemistry.
From www.sarthaks.com
The energy profile diagram for the reaction `CO(g)+NO_(2)(g) hArr CO What Is A Threshold Energy In Chemistry For a chemical reaction to occur, an energy threshold must be. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. The difference between these two values is the activation. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react. What Is A Threshold Energy In Chemistry.
From byjus.com
What happens when the energy of a reaction if more than threshold What Is A Threshold Energy In Chemistry the threshold energy is the energy that these molecules must have in order for a reaction to take place. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. This equation is valid if the energies are much less than rest mass energies of the involved particles. calculate the threshold energy. What Is A Threshold Energy In Chemistry.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent What Is A Threshold Energy In Chemistry In other words, the kinetic. activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. This equation is valid if the energies are much less than rest mass energies of the involved particles. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for. What Is A Threshold Energy In Chemistry.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical What Is A Threshold Energy In Chemistry the threshold energy is the energy that these molecules must have in order for a reaction to take place. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant. For a chemical reaction to occur, an energy threshold must be. activation energy is primarily associated with chemical reactions,. What Is A Threshold Energy In Chemistry.
From guidefixcasiranilv.z4.web.core.windows.net
How To Read Energy Diagrams Chemistry What Is A Threshold Energy In Chemistry calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions. What Is A Threshold Energy In Chemistry.
From www.meritnation.com
what is the formula of threshold energy Chemistry Structure of Atom What Is A Threshold Energy In Chemistry For a chemical reaction to occur, an energy threshold must be. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant. This equation is valid if the energies are much less than rest mass energies of the involved particles. The difference between these two values is the activation. the. What Is A Threshold Energy In Chemistry.
From www.youtube.com
What is activation energy Threshold energy energy barrier rate of What Is A Threshold Energy In Chemistry The difference between these two values is the activation. This equation is valid if the energies are much less than rest mass energies of the involved particles. For a chemical reaction to occur, an energy threshold must be. the threshold energy is the energy that these molecules must have in order for a reaction to take place. activation. What Is A Threshold Energy In Chemistry.
From www.science-revision.co.uk
Energy profile diagrams What Is A Threshold Energy In Chemistry activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. The difference between. What Is A Threshold Energy In Chemistry.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions What Is A Threshold Energy In Chemistry the threshold energy is the energy that these molecules must have in order for a reaction to take place. calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric. In other words, the kinetic. For a chemical reaction to occur, an energy threshold must be. This equation is. What Is A Threshold Energy In Chemistry.
From jbapasd.blogspot.com
Energy Level Diagram Chemistry Patterns and Trends in the Periodic What Is A Threshold Energy In Chemistry the threshold energy is the energy that these molecules must have in order for a reaction to take place. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when. What Is A Threshold Energy In Chemistry.
From byjus.com
Reaction Coordinate Diagram An Overview of Reaction Coordinate What Is A Threshold Energy In Chemistry In other words, the kinetic. The difference between these two values is the activation. This equation is valid if the energies are much less than rest mass energies of the involved particles. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. activation energy is primarily associated. What Is A Threshold Energy In Chemistry.
From www.expii.com
Energy Diagram — Overview & Parts Expii What Is A Threshold Energy In Chemistry in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. In other words, the kinetic. The difference between these two values is the activation. For a chemical reaction to occur, an energy threshold must be. calculate the threshold energy in kj/mol of electrons in aluminum, given that. What Is A Threshold Energy In Chemistry.
From byjus.com
What is the effect of catalyst on threshold energy? What Is A Threshold Energy In Chemistry This equation is valid if the energies are much less than rest mass energies of the involved particles. The difference between these two values is the activation. For a chemical reaction to occur, an energy threshold must be. activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. threshold energy. What Is A Threshold Energy In Chemistry.
From www.youtube.com
Explain the terms Threshold energy 12 CHEMICAL What Is A Threshold Energy In Chemistry the threshold energy is the energy that these molecules must have in order for a reaction to take place. The difference between these two values is the activation. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant. collision theory provides a qualitative explanation of chemical reactions and. What Is A Threshold Energy In Chemistry.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the What Is A Threshold Energy In Chemistry activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform. in chemical reactions, the energy barrier corresponds to the amount of energy the particles must have to react when they collide. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. For a. What Is A Threshold Energy In Chemistry.