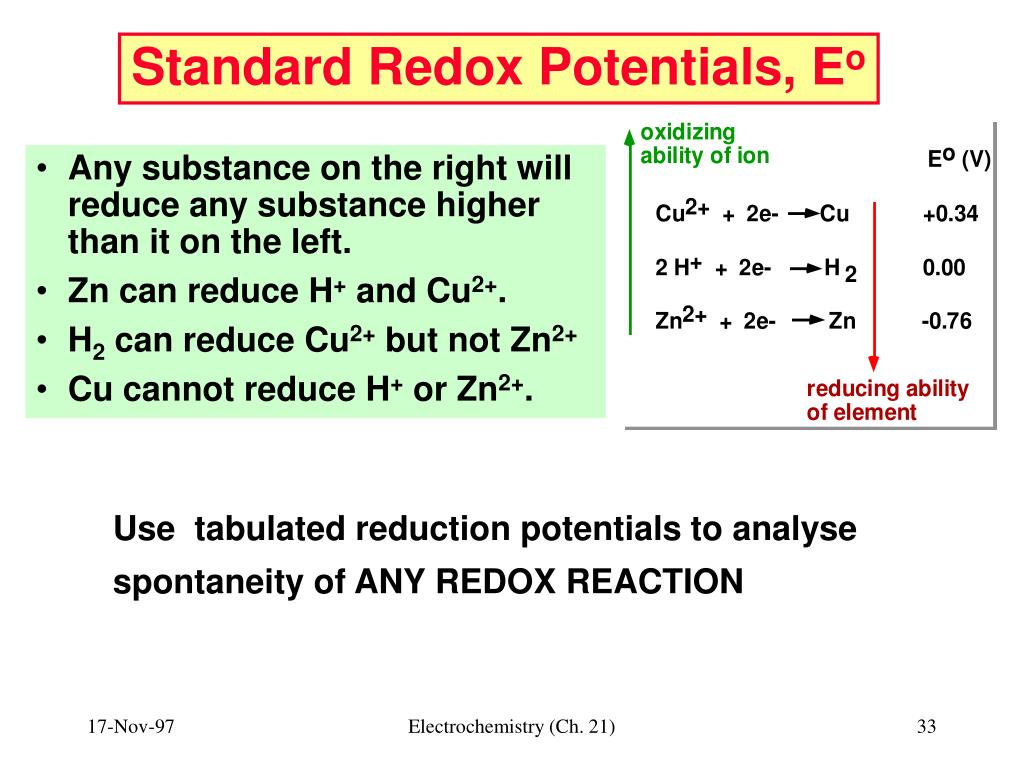
Standard Reduction Potential Mercury . Values are from the following sources: 183 rows the following table provides eo for selected reduction reactions. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm). Use standard reduction potentials to determine the better oxidizing or. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Standard reduction potentials by element. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. Use standard reduction potentials to determine the better. Table 1 is an alphabetical. The following table provides eo and eo ´ values for selected reduction reactions. 45 rows standard electrode potentials in aqueous solution at 25°c.
from www.slideserve.com
Table 1 is an alphabetical. 183 rows the following table provides eo for selected reduction reactions. 45 rows standard electrode potentials in aqueous solution at 25°c. Standard reduction potentials by element. The following table provides eo and eo ´ values for selected reduction reactions. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm). The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. Values are from the following sources: Use standard reduction potentials to determine the better. Use standard reduction potentials to determine the better oxidizing or.
PPT ELECTROCHEMISTRY Chapter 21 PowerPoint Presentation, free download ID812189
Standard Reduction Potential Mercury 183 rows the following table provides eo for selected reduction reactions. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Use standard reduction potentials to determine the better oxidizing or. Values are from the following sources: Table 1 is an alphabetical. 183 rows the following table provides eo for selected reduction reactions. Use standard reduction potentials to determine the better. Standard reduction potentials by element. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm). The following table provides eo and eo ´ values for selected reduction reactions. 45 rows standard electrode potentials in aqueous solution at 25°c.
From pressbooks.uiowa.edu
Topic 11 Appendix A Standard Reduction Potentials at 25ºC CHEM 1120 Lab Manual Standard Reduction Potential Mercury The following table provides eo and eo ´ values for selected reduction reactions. Use standard reduction potentials to determine the better oxidizing or. Use standard reduction potentials to determine the better. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. Each. Standard Reduction Potential Mercury.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Mercury Values are from the following sources: 45 rows standard electrode potentials in aqueous solution at 25°c. The following table provides eo and eo ´ values for selected reduction reactions. Table 1 is an alphabetical. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for. Standard Reduction Potential Mercury.
From oneclass.com
OneClass Using standard reduction potentials from the ALEKS Data tab calculate the standard... Standard Reduction Potential Mercury Standard reduction potentials by element. The following table provides eo and eo ´ values for selected reduction reactions. Use standard reduction potentials to determine the better oxidizing or. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Table 1 is an alphabetical. Use standard reduction potentials to determine. Standard Reduction Potential Mercury.
From www.flinnsci.ca
Standard Reduction Potential Chart Flinn Scientific Standard Reduction Potential Mercury Use standard reduction potentials to determine the better. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm). Standard reduction potentials by element. Values are from the following sources: 45 rows standard electrode potentials in aqueous solution at 25°c. Table 1 is an alphabetical. The following table provides. Standard Reduction Potential Mercury.
From chemwiki.ucdavis.edu
Standard Potentials Chemwiki Standard Reduction Potential Mercury Values are from the following sources: The following table provides eo and eo ´ values for selected reduction reactions. Table 1 is an alphabetical. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm). Use standard reduction potentials to determine the better. The standard reduction potential can be. Standard Reduction Potential Mercury.
From studylib.net
Standard Reduction Potentials Standard Reduction Potential Mercury Use standard reduction potentials to determine the better oxidizing or. Use standard reduction potentials to determine the better. Table 1 is an alphabetical. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm). The following table provides eo and eo ´ values for selected reduction reactions. Values are. Standard Reduction Potential Mercury.
From ch302.cm.utexas.edu
Standard Reduction Potentials Standard Reduction Potential Mercury Use standard reduction potentials to determine the better. The following table provides eo and eo ´ values for selected reduction reactions. 45 rows standard electrode potentials in aqueous solution at 25°c. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm). 372 rows the data below tabulates standard. Standard Reduction Potential Mercury.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID541675 Standard Reduction Potential Mercury The following table provides eo and eo ´ values for selected reduction reactions. 45 rows standard electrode potentials in aqueous solution at 25°c. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. 183 rows the following table provides eo for selected. Standard Reduction Potential Mercury.
From testbook.com
Reduction Potential Measurement, Half Cells, Differences & More Standard Reduction Potential Mercury Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm). 183 rows the following table provides eo for selected reduction reactions. The following table provides eo and eo ´ values for selected reduction reactions. Standard reduction potentials by element. Values are from the following sources: 372 rows the. Standard Reduction Potential Mercury.
From f15.beauty
Standard Reduction Potential Table Standard Reduction Potential Mercury The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. Table 1 is an alphabetical. Use standard reduction potentials to determine the better. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode. Standard Reduction Potential Mercury.
From www.pinterest.com
Standard Reduction Potentials Reduction potential, Equations, Chemistry lessons Standard Reduction Potential Mercury Table 1 is an alphabetical. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 183 rows the following table provides eo for selected reduction reactions. Use standard reduction potentials to determine the better oxidizing or. The following table provides eo and eo ´ values for selected reduction reactions.. Standard Reduction Potential Mercury.
From www.studypool.com
SOLUTION Table of standard reduction potentials 1 Studypool Standard Reduction Potential Mercury 45 rows standard electrode potentials in aqueous solution at 25°c. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm).. Standard Reduction Potential Mercury.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Mercury The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. The following table provides eo and eo ´ values for selected reduction reactions. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325. Standard Reduction Potential Mercury.
From www.slideserve.com
PPT Transition Metals & Coordination Chemistry PowerPoint Presentation ID224645 Standard Reduction Potential Mercury Use standard reduction potentials to determine the better oxidizing or. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The following table provides eo and eo ´ values for selected reduction reactions. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a. Standard Reduction Potential Mercury.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free download ID5480021 Standard Reduction Potential Mercury The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. 183 rows the following table provides eo for selected reduction reactions. Table 1 is an alphabetical. Values are from the following sources: Each table lists standard reduction potentials, e° values, at 298.15. Standard Reduction Potential Mercury.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free download ID4491322 Standard Reduction Potential Mercury The following table provides eo and eo ´ values for selected reduction reactions. Use standard reduction potentials to determine the better. Table 1 is an alphabetical. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm). The standard reduction potential can be determined by subtracting the standard reduction. Standard Reduction Potential Mercury.
From www.scribd.com
Table of Standard Reduction Potential PDF Standard Reduction Potential Mercury Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. Values are from the following sources: Standard reduction potentials by element. Each table lists standard reduction potentials, e° values, at 298.15 k. Standard Reduction Potential Mercury.
From www.chegg.com
Solved 5. (a) Use the standard reduction potentials at 25° C Standard Reduction Potential Mercury 45 rows standard electrode potentials in aqueous solution at 25°c. Standard reduction potentials by element. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. Table 1 is an alphabetical. 183 rows the following table provides eo for selected reduction reactions. Values. Standard Reduction Potential Mercury.
From www.slideserve.com
PPT Chapter 20 Electron Transfer Reactions PowerPoint Presentation ID1951700 Standard Reduction Potential Mercury Table 1 is an alphabetical. Values are from the following sources: The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. Standard reduction potentials by element. 183 rows the following table provides eo for selected reduction reactions. The following table provides eo. Standard Reduction Potential Mercury.
From www.youtube.com
Standard Reduction Potential YouTube Standard Reduction Potential Mercury 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Table 1 is an alphabetical. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm). Use standard reduction potentials to determine the better. 45 rows standard electrode. Standard Reduction Potential Mercury.
From mavink.com
Standard Reduction Potentials Standard Reduction Potential Mercury Use standard reduction potentials to determine the better oxidizing or. Standard reduction potentials by element. Table 1 is an alphabetical. 183 rows the following table provides eo for selected reduction reactions. Values are from the following sources: 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard. Standard Reduction Potential Mercury.
From www.slideserve.com
PPT ELECTROCHEMISTRY Chapter 21 PowerPoint Presentation, free download ID812189 Standard Reduction Potential Mercury 183 rows the following table provides eo for selected reduction reactions. 45 rows standard electrode potentials in aqueous solution at 25°c. Use standard reduction potentials to determine the better. Table 1 is an alphabetical. Values are from the following sources: 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she),. Standard Reduction Potential Mercury.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials Chemistry Standard Reduction Potential Mercury Standard reduction potentials by element. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Table 1 is an alphabetical. Use standard reduction potentials to determine the better. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from. Standard Reduction Potential Mercury.
From loensgcfn.blob.core.windows.net
Standard Reduction Potential Calculator at James Burkley blog Standard Reduction Potential Mercury Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm). Use standard reduction potentials to determine the better. Use standard reduction potentials to determine the better oxidizing or. Values are from the following sources: Table 1 is an alphabetical. The following table provides eo and eo ´ values. Standard Reduction Potential Mercury.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Mercury 183 rows the following table provides eo for selected reduction reactions. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 45 rows standard electrode potentials in aqueous solution at 25°c. The following table provides eo and eo ´ values for selected reduction reactions. Standard reduction potentials by element.. Standard Reduction Potential Mercury.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu Standard Reduction Potential Mercury 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. Table 1 is an alphabetical. Use standard reduction potentials to determine. Standard Reduction Potential Mercury.
From www.chem.fsu.edu
Lab Purpose Standard Reduction Potential Mercury 183 rows the following table provides eo for selected reduction reactions. Use standard reduction potentials to determine the better. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. 372 rows the data below tabulates standard electrode potentials (e °), in volts. Standard Reduction Potential Mercury.
From www.slideserve.com
PPT Oxidation and Reduction PowerPoint Presentation, free download ID4653489 Standard Reduction Potential Mercury 45 rows standard electrode potentials in aqueous solution at 25°c. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm). Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Standard Reduction Potential Mercury.
From www.researchgate.net
Standard reduction potentials versus SHE; data from references [89,91]. Download Scientific Standard Reduction Potential Mercury Standard reduction potentials by element. 45 rows standard electrode potentials in aqueous solution at 25°c. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure. Standard Reduction Potential Mercury.
From www.slideserve.com
PPT Product stabilizations in hydrolysis PowerPoint Presentation, free download ID3696784 Standard Reduction Potential Mercury The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at the anode from the standard reduction potential for the reaction. Table 1 is an alphabetical. Use standard reduction potentials to determine the better oxidizing or. Values are from the following sources: Each table lists standard reduction potentials, e° values, at 298.15 k. Standard Reduction Potential Mercury.
From www.researchgate.net
Standard reduction potentials (E o ) of some reactions at 25 o C [21,22]. Download Scientific Standard Reduction Potential Mercury Use standard reduction potentials to determine the better. Values are from the following sources: Use standard reduction potentials to determine the better oxidizing or. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Table 1 is an alphabetical. Each table lists standard reduction potentials, e° values, at 298.15. Standard Reduction Potential Mercury.
From www.slideserve.com
PPT Redox Reactions and Electrochemistry PowerPoint Presentation, free download ID4399853 Standard Reduction Potential Mercury 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Use standard reduction potentials to determine the better oxidizing or. Standard reduction potentials by element. Use standard reduction potentials to determine the better. 45 rows standard electrode potentials in aqueous solution at 25°c. The standard reduction potential can be. Standard Reduction Potential Mercury.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free download ID5480021 Standard Reduction Potential Mercury The following table provides eo and eo ´ values for selected reduction reactions. 183 rows the following table provides eo for selected reduction reactions. Use standard reduction potentials to determine the better. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1 atm). 45 rows standard electrode potentials in. Standard Reduction Potential Mercury.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts Standard Reduction Potential Mercury Table 1 is an alphabetical. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The following table provides eo and eo ´ values for selected reduction reactions. 45 rows standard electrode potentials in aqueous solution at 25°c. The standard reduction potential can be determined by subtracting the standard. Standard Reduction Potential Mercury.
From slideplayer.com
Electrochemistry Part III Reduction Potentials ppt download Standard Reduction Potential Mercury The following table provides eo and eo ´ values for selected reduction reactions. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Table 1 is an alphabetical. Each table lists standard reduction potentials, e° values, at 298.15 k (25 °c), and at a pressure of 101.325 kpa (1. Standard Reduction Potential Mercury.