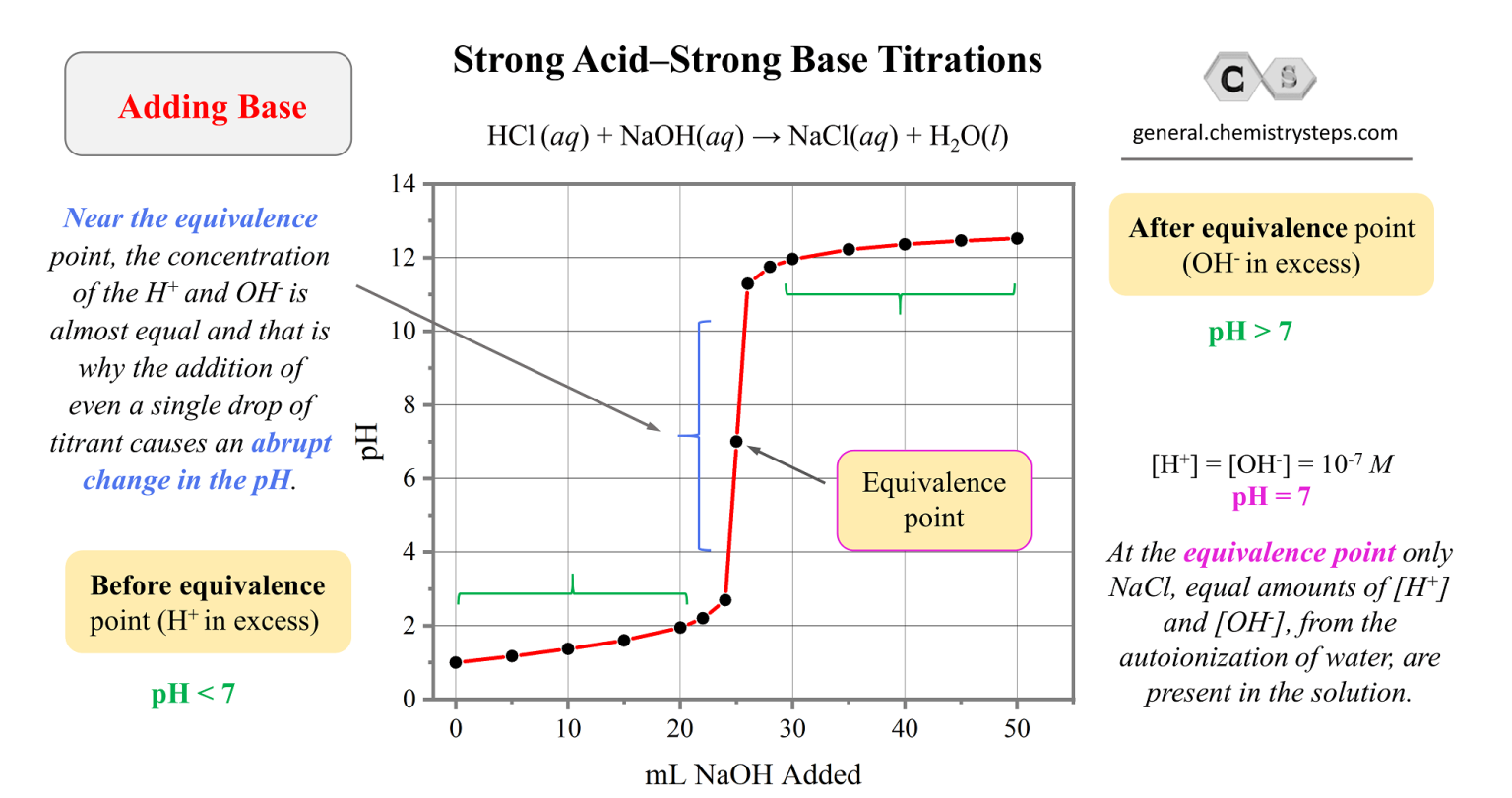
Titration Problems Acid Base . There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an. Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. Comment on the trend of ph change in the titration between a weak acid and weak base. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. It is based on the neutralization reaction , where. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. In an indicator based titration you add another chemical that changes color at the ph equal.
from general.chemistrysteps.com
Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. Comment on the trend of ph change in the titration between a weak acid and weak base. It is based on the neutralization reaction , where. In an indicator based titration you add another chemical that changes color at the ph equal. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. There are two basic types of acid base titrations, indicator and potentiometric. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The analyte (titrand) is the solution with an.
Strong AcidStrong Base Titrations Chemistry Steps
Titration Problems Acid Base Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. There are two basic types of acid base titrations, indicator and potentiometric. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The analyte (titrand) is the solution with an. Comment on the trend of ph change in the titration between a weak acid and weak base. It is based on the neutralization reaction , where. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. In an indicator based titration you add another chemical that changes color at the ph equal.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Problems Acid Base In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. In an indicator based titration you add another chemical that changes. Titration Problems Acid Base.
From www.youtube.com
Acidbase titration problem 1 YouTube Titration Problems Acid Base In an indicator based titration you add another chemical that changes color at the ph equal. Comment on the trend of ph change in the titration between a weak acid and weak base. There are two basic types of acid base titrations, indicator and potentiometric. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used. Titration Problems Acid Base.
From mungfali.com
Acid Base Titration Procedure Titration Problems Acid Base Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. Comment on the trend of ph change in the titration between a weak acid and weak base. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a. Titration Problems Acid Base.
From www.studypool.com
SOLUTION Acid base titration solved problems Studypool Titration Problems Acid Base Comment on the trend of ph change in the titration between a weak acid and weak base. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. It is based on the neutralization reaction , where. The analyte (titrand) is the solution with an. There are two. Titration Problems Acid Base.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Problems Acid Base In an indicator based titration you add another chemical that changes color at the ph equal. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. It is based on the neutralization reaction , where. The analyte (titrand) is the solution with an. Example 10 is the. Titration Problems Acid Base.
From www.chemistrylearner.com
Free Printable Acids and Bases Titration Worksheets Titration Problems Acid Base It is based on the neutralization reaction , where. Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The analyte. Titration Problems Acid Base.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Titration Problems Acid Base It is based on the neutralization reaction , where. Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. The analyte (titrand) is the solution with an. Comment on the trend of ph change in the titration between a weak acid and weak base. As seen in the chapter. Titration Problems Acid Base.
From www.chegg.com
Solved AcidBase Titration Practice Problems 1) Calculate Titration Problems Acid Base The analyte (titrand) is the solution with an. In an indicator based titration you add another chemical that changes color at the ph equal. Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. It is based on the neutralization reaction , where. As seen in the chapter on. Titration Problems Acid Base.
From www.docsity.com
Titration Practice Acid Base Reaction Worksheet with Answer Key Docsity Titration Problems Acid Base Comment on the trend of ph change in the titration between a weak acid and weak base. It is based on the neutralization reaction , where. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an. Example 10 is the titration of the salt of a weak acid (making the. Titration Problems Acid Base.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Problems Acid Base Comment on the trend of ph change in the titration between a weak acid and weak base. The analyte (titrand) is the solution with an. Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. Titration Problems Acid Base.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Problems Acid Base There are two basic types of acid base titrations, indicator and potentiometric. Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. In an indicator based titration you add another chemical that changes color at the ph equal. As seen in the chapter on the stoichiometry of chemical reactions,. Titration Problems Acid Base.
From www.tes.com
Acid Base Titrations, Neutralization, Equivalence Point Grade 11 Titration Problems Acid Base In an indicator based titration you add another chemical that changes color at the ph equal. The analyte (titrand) is the solution with an. There are two basic types of acid base titrations, indicator and potentiometric. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. In. Titration Problems Acid Base.
From studylib.net
ACIDBASE TITRATION Titration Problems Acid Base In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. In an indicator based titration you add another chemical. Titration Problems Acid Base.
From studylib.net
Chapter 10 AcidBase titrations Titration Problems Acid Base It is based on the neutralization reaction , where. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. In an indicator based titration you add another chemical that changes color at the ph equal. Comment on the trend of ph change. Titration Problems Acid Base.
From mavink.com
Acid Base Titration Equation Titration Problems Acid Base The analyte (titrand) is the solution with an. Comment on the trend of ph change in the titration between a weak acid and weak base. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. In this section, we will see how to perform calculations to predict. Titration Problems Acid Base.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Problems Acid Base As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Comment on the trend of ph change in the titration between a weak acid and weak base. There are two basic types of acid base titrations, indicator and potentiometric. In this section, we will see how to. Titration Problems Acid Base.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration Problems Acid Base Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. It is based on the neutralization reaction , where. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. The analyte (titrand) is the solution with. Titration Problems Acid Base.
From www.youtube.com
acids and bases examples of titration problems YouTube Titration Problems Acid Base In an indicator based titration you add another chemical that changes color at the ph equal. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Example 10 is the titration of the salt of a weak acid (making the salt a. Titration Problems Acid Base.
From studylib.net
acid base titration worksheet answer key Titration Problems Acid Base Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. It is based on the neutralization reaction , where. In an. Titration Problems Acid Base.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titration Problems Acid Base In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. In an indicator based titration you add another chemical that changes color at the ph equal. The analyte (titrand) is the solution with an. Example 10 is the titration of the salt. Titration Problems Acid Base.
From tukioka-clinic.com
️ Acid base titration problems with answers pdf. Eleventh grade Lesson Titration Problems Acid Base It is based on the neutralization reaction , where. The analyte (titrand) is the solution with an. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used. Titration Problems Acid Base.
From www.studypool.com
SOLUTION Acid base equilibria and titration solved problems Studypool Titration Problems Acid Base Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. There are two basic types of acid base titrations, indicator and potentiometric. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. In an indicator based. Titration Problems Acid Base.
From mavink.com
Acid Base Titration Problems Titration Problems Acid Base There are two basic types of acid base titrations, indicator and potentiometric. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. In an indicator based titration you add another chemical that changes color at the ph equal. As seen in the. Titration Problems Acid Base.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Titration Problems Acid Base In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. There are two basic types of acid base titrations,. Titration Problems Acid Base.
From www.youtube.com
5 Solving Titration problems with acids and bases YouTube Titration Problems Acid Base Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Comment on the trend of ph change in the titration between a weak acid and weak. Titration Problems Acid Base.
From mavink.com
Acid Base Titration Calculation Titration Problems Acid Base Comment on the trend of ph change in the titration between a weak acid and weak base. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an. It is based on the neutralization reaction , where. Example 10 is the titration of the salt of a weak acid (making the. Titration Problems Acid Base.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration Problems Acid Base It is based on the neutralization reaction , where. In an indicator based titration you add another chemical that changes color at the ph equal. Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. There are two basic types of acid base titrations, indicator and potentiometric. Comment on. Titration Problems Acid Base.
From www.studypool.com
SOLUTION Acid Base Titration Datasheet Studypool Titration Problems Acid Base There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal. The analyte (titrand) is the solution with an. Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. It is based. Titration Problems Acid Base.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Problems Acid Base There are two basic types of acid base titrations, indicator and potentiometric. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. It is based on the neutralization reaction , where. As seen in the chapter on the stoichiometry of chemical reactions,. Titration Problems Acid Base.
From mungfali.com
Acid Base Titration Calculation Titration Problems Acid Base Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid. Comment on the trend of ph change in the titration between a weak acid and weak base. In an indicator based titration you add another chemical that changes color at the ph equal. The analyte (titrand) is the solution. Titration Problems Acid Base.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Problems Acid Base In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Comment on the trend of ph change in the titration between a weak acid and weak base. It is based on the neutralization reaction , where. As seen in the chapter on. Titration Problems Acid Base.
From www.youtube.com
Strong AcidStrong Base Titration Problem (Chemwiki Solution) YouTube Titration Problems Acid Base In an indicator based titration you add another chemical that changes color at the ph equal. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. In this section, we will see how to perform calculations to predict the ph at any point in a titration of. Titration Problems Acid Base.
From www.slideserve.com
PPT Unit 7 PowerPoint Presentation, free download ID5948396 Titration Problems Acid Base In an indicator based titration you add another chemical that changes color at the ph equal. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid.. Titration Problems Acid Base.
From www.youtube.com
Acid Base titration problem solving YouTube Titration Problems Acid Base In an indicator based titration you add another chemical that changes color at the ph equal. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Example 10 is the titration of the salt of a weak acid (making the salt a base) with a strong acid.. Titration Problems Acid Base.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Problems Acid Base There are two basic types of acid base titrations, indicator and potentiometric. It is based on the neutralization reaction , where. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. As seen in the chapter on the stoichiometry of chemical reactions,. Titration Problems Acid Base.