What Does A High Vapor Pressure Mean . Volatile liquids are liquids with high. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); If the intermolecular forces are small, the liquid has a high vapour pressure. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. That is, the pressure of the vapor resulting from. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Little heat energy will have to be added to separate.
from www.slideserve.com
Volatile liquids are liquids with high. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. That is, the pressure of the vapor resulting from. Little heat energy will have to be added to separate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. If the intermolecular forces are small, the liquid has a high vapour pressure.
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735
What Does A High Vapor Pressure Mean Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. That is, the pressure of the vapor resulting from. Volatile liquids are liquids with high. Little heat energy will have to be added to separate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); If the intermolecular forces are small, the liquid has a high vapour pressure.
From slideplayer.com
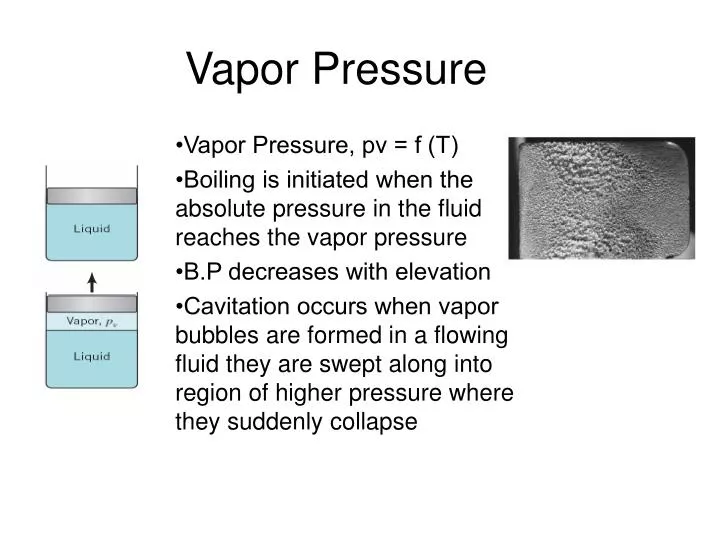
Vapor Pressure Vaporization change from liquid to gas at boiling What Does A High Vapor Pressure Mean If the intermolecular forces are small, the liquid has a high vapour pressure. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Volatile liquids are liquids with high. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured. What Does A High Vapor Pressure Mean.
From www.iqsdirectory.com
Ultrasonic Cleaning What Is It? How Does It Work? Types Of What Does A High Vapor Pressure Mean That is, the pressure of the vapor resulting from. Volatile liquids are liquids with high. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Little heat energy will have to be added to separate. If the. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID245553 What Does A High Vapor Pressure Mean Little heat energy will have to be added to separate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. If the intermolecular forces are small, the liquid has a high vapour pressure. That is, the pressure of the vapor resulting from. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium. What Does A High Vapor Pressure Mean.
From vacaero.com
Simple Physics for the High Vacuum Processing Industry What Does A High Vapor Pressure Mean Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. That is, the pressure of the vapor resulting from. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Volatile liquids are liquids with high. The vapor pressure of a liquid. What Does A High Vapor Pressure Mean.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube What Does A High Vapor Pressure Mean The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. If the intermolecular. What Does A High Vapor Pressure Mean.
From study.com
Vapor Pressure Definition, Equation & Examples Video & Lesson What Does A High Vapor Pressure Mean That is, the pressure of the vapor resulting from. Volatile liquids are liquids with high. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Little heat energy will have to be added to separate. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or. What Does A High Vapor Pressure Mean.
From byjus.com
What does the following statement mean? "A liquid having weaker What Does A High Vapor Pressure Mean This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Little heat energy will have to be added to separate. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Volatile liquids. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Does A High Vapor Pressure Mean Volatile liquids are liquids with high. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Little heat energy will have to be added to separate. That is, the pressure of. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Lecture 7 PowerPoint Presentation, free download ID3933711 What Does A High Vapor Pressure Mean Volatile liquids are liquids with high. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); This equation can be used to calculate the enthalpy of vaporization of. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 What Does A High Vapor Pressure Mean If the intermolecular forces are small, the liquid has a high vapour pressure. That is, the pressure of the vapor resulting from. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the. What Does A High Vapor Pressure Mean.
From www.youtube.com
Which Compound Has a Higher Vapor Pressure? Intermolecular Force What Does A High Vapor Pressure Mean The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Volatile liquids are liquids with high. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. If the intermolecular forces are small, the liquid has a high vapour pressure. That is, the pressure of the vapor resulting. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 What Does A High Vapor Pressure Mean That is, the pressure of the vapor resulting from. Little heat energy will have to be added to separate. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This equation can be used to calculate the. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free What Does A High Vapor Pressure Mean If the intermolecular forces are small, the liquid has a high vapour pressure. That is, the pressure of the vapor resulting from. Little heat energy will have to be added to separate. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Vapour pressure, pressure exerted. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint What Does A High Vapor Pressure Mean Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. If the intermolecular forces are small, the liquid has a high vapour pressure. Little heat energy will have to be added to separate. Volatile liquids are liquids with high. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid. What Does A High Vapor Pressure Mean.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Does A High Vapor Pressure Mean If the intermolecular forces are small, the liquid has a high vapour pressure. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. That is, the pressure of the vapor resulting from. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the. What Does A High Vapor Pressure Mean.
From socratic.org
What do we mean by a dynamic equilibrium? Can you describe how the What Does A High Vapor Pressure Mean Volatile liquids are liquids with high. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. That is, the pressure of the vapor resulting from. This equation can. What Does A High Vapor Pressure Mean.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Does A High Vapor Pressure Mean Little heat energy will have to be added to separate. Volatile liquids are liquids with high. If the intermolecular forces are small, the liquid has a high vapour pressure. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from. Vapour pressure, pressure exerted. What Does A High Vapor Pressure Mean.
From ar.inspiredpencil.com
Vapor Pressure Example What Does A High Vapor Pressure Mean Volatile liquids are liquids with high. Little heat energy will have to be added to separate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. That is, the pressure of. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation ID352441 What Does A High Vapor Pressure Mean That is, the pressure of the vapor resulting from. Volatile liquids are liquids with high. Little heat energy will have to be added to separate. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. This equation. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 What Does A High Vapor Pressure Mean Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); If the intermolecular forces are small, the liquid has a high vapour pressure. That is, the pressure of. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 What Does A High Vapor Pressure Mean That is, the pressure of the vapor resulting from. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. If the intermolecular forces are small, the liquid has a high vapour. What Does A High Vapor Pressure Mean.
From saylordotorg.github.io
Vapor Pressure What Does A High Vapor Pressure Mean Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Little heat energy will have to be added to separate. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. If the intermolecular forces are small, the liquid has a high. What Does A High Vapor Pressure Mean.
From in.pinterest.com
Vapor Pressure Easy Science Chemistry education, Learn biology What Does A High Vapor Pressure Mean That is, the pressure of the vapor resulting from. Little heat energy will have to be added to separate. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or. What Does A High Vapor Pressure Mean.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Does A High Vapor Pressure Mean Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Volatile liquids are liquids with high. This equation can be used to calculate the enthalpy of vaporization of. What Does A High Vapor Pressure Mean.
From brunofuga.adv.br
Vapor Pressure Definition And How To Calculate It, 51 OFF What Does A High Vapor Pressure Mean The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Little heat energy will have to be added to separate. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. That is, the pressure of the vapor resulting. What Does A High Vapor Pressure Mean.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Does A High Vapor Pressure Mean The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. If the intermolecular forces are small, the liquid has a high vapour pressure. Little heat energy will have. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2965441 What Does A High Vapor Pressure Mean Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Little heat energy will have to be added to separate. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Volatile liquids are liquids with high. The vapor pressure of a. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735 What Does A High Vapor Pressure Mean Little heat energy will have to be added to separate. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. If the intermolecular forces are small, the liquid has a high vapour pressure. Volatile liquids are liquids. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2237600 What Does A High Vapor Pressure Mean This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. That is, the pressure of the vapor resulting from. Volatile liquids are liquids with high. Little heat energy will have to be added to separate. If the intermolecular forces are small, the liquid has a high. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download What Does A High Vapor Pressure Mean Little heat energy will have to be added to separate. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Generally a substance's vapor pressure increases as temperature. What Does A High Vapor Pressure Mean.
From studylib.net
Vapor Pressure of Solutions What Does A High Vapor Pressure Mean Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Generally a substance's vapor pressure increases as temperature increases and decreases as. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Vapor Pressure Lowering PowerPoint Presentation, free download What Does A High Vapor Pressure Mean Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatile liquids are liquids with high. Vapour pressure, pressure exerted by a vapour when the vapour is in equilibrium with the liquid or solid form, or both, of the same. Little heat energy will have to be added to separate. The vapor pressure of a. What Does A High Vapor Pressure Mean.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Does A High Vapor Pressure Mean Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); If the intermolecular forces are small, the liquid has a high vapour pressure. That is, the pressure of the vapor resulting from. Volatile liquids are liquids with. What Does A High Vapor Pressure Mean.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Does A High Vapor Pressure Mean Volatile liquids are liquids with high. This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. If the intermolecular forces are small, the liquid has a high vapour pressure. Little heat. What Does A High Vapor Pressure Mean.
From socratic.org
Explain how each of the following affects the vapour pressure of a What Does A High Vapor Pressure Mean If the intermolecular forces are small, the liquid has a high vapour pressure. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); This equation can be used to calculate the enthalpy of vaporization of a liquid from its measured vapor pressure at two or more temperatures. Little heat energy will have. What Does A High Vapor Pressure Mean.