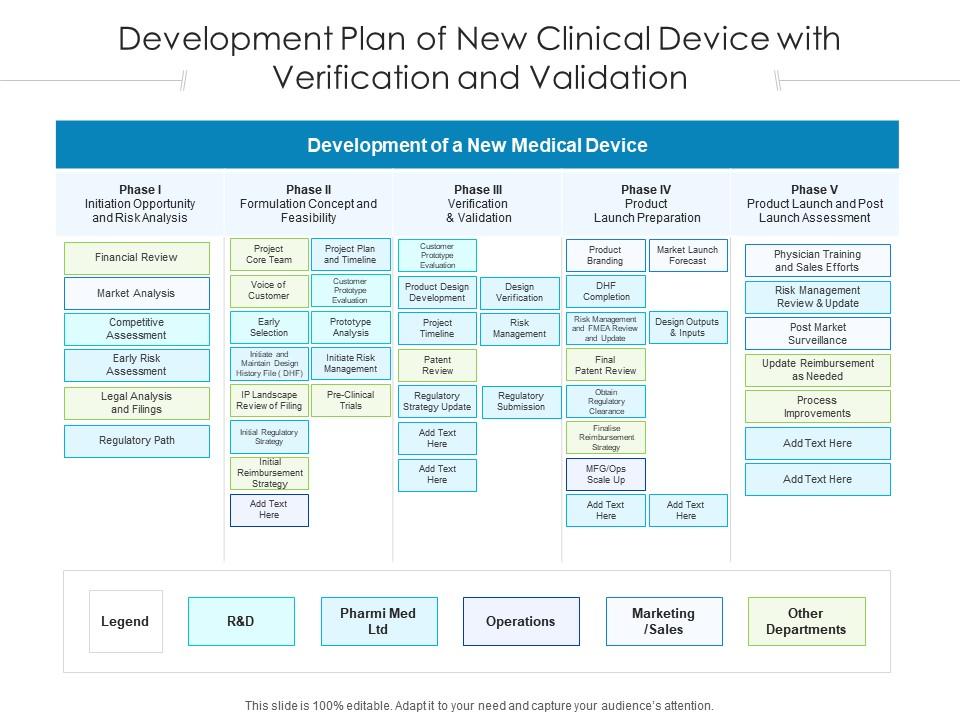
Dhf Medical Device Template . Explore the essential role of the design history file (dhf) in ensuring compliance, quality, and. In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. Here’s what you need to know about the dhf and its ongoing importance in fda compliance. Its purpose is to demonstrate that the device. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: The design history file (dhf): A guide to compiling a dhf for medical device development. Is a collection of documents that describe the design and development activities of a medical device. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. But that doesn’t mean the requirement is being dropped. A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design.
from prntbl.concejomunicipaldechinu.gov.co
A guide to compiling a dhf for medical device development. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Here’s what you need to know about the dhf and its ongoing importance in fda compliance. A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. Its purpose is to demonstrate that the device. Is a collection of documents that describe the design and development activities of a medical device. Explore the essential role of the design history file (dhf) in ensuring compliance, quality, and. In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. But that doesn’t mean the requirement is being dropped. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026.
Medical Device Verification And Validation Plan Template prntbl
Dhf Medical Device Template Is a collection of documents that describe the design and development activities of a medical device. A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. But that doesn’t mean the requirement is being dropped. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Its purpose is to demonstrate that the device. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. Here’s what you need to know about the dhf and its ongoing importance in fda compliance. A guide to compiling a dhf for medical device development. The design history file (dhf): Explore the essential role of the design history file (dhf) in ensuring compliance, quality, and. Is a collection of documents that describe the design and development activities of a medical device.
From www.cognidox.com
Compiling a Design History File (DHF) for a medical device product Dhf Medical Device Template Here’s what you need to know about the dhf and its ongoing importance in fda compliance. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Is a collection of documents that describe the design and development activities of a medical device. Its purpose is to demonstrate that the device.. Dhf Medical Device Template.
From www.spkaa.com
The Trio of Documentation DHF, DMR, and DHR in Medical Device Dhf Medical Device Template But that doesn’t mean the requirement is being dropped. Its purpose is to demonstrate that the device. A guide to compiling a dhf for medical device development. Explore the essential role of the design history file (dhf) in ensuring compliance, quality, and. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect. Dhf Medical Device Template.
From www.qualio.com
Assembling a Design History File (DHF) for your medical device Dhf Medical Device Template Is a collection of documents that describe the design and development activities of a medical device. In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in. Dhf Medical Device Template.
From template.mapadapalavra.ba.gov.br
Medical Device Design And Development Plan Template Dhf Medical Device Template The design history file (dhf): Its purpose is to demonstrate that the device. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: A guide to compiling a dhf for medical device development. But that doesn’t mean the requirement is being dropped. The fda’s new qmsr is dropping the reference to the. Dhf Medical Device Template.
From www.scilife.io
What is Design History File (DHF)? Complete definition Scilife Dhf Medical Device Template This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. Explore the essential role of the design history file (dhf) in ensuring compliance, quality, and. The fda’s. Dhf Medical Device Template.
From www.spkaa.com
The Trio of Documentation DHF, DMR, and DHR in Medical Device Dhf Medical Device Template A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. But that doesn’t mean the requirement is being dropped. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Explore the essential role of the design history file (dhf) in. Dhf Medical Device Template.
From khanzafathiaulia.blogspot.com
Medical Device File Vs Dmr khanzafathiaulia Dhf Medical Device Template Its purpose is to demonstrate that the device. But that doesn’t mean the requirement is being dropped. Is a collection of documents that describe the design and development activities of a medical device. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: A dhf template could be created to follow a. Dhf Medical Device Template.
From www.qualio.com
Assembling a Design History File (DHF) for your medical device Dhf Medical Device Template The design history file (dhf): In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. Here’s what you need to know about the dhf and its ongoing importance in fda compliance. But that doesn’t mean the requirement is being dropped. This white paper focuses on. Dhf Medical Device Template.
From informacionpublica.svet.gob.gt
Assembling A Design History File (DHF) For Your Medical Dhf Medical Device Template The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. The design history file (dhf): In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. But that doesn’t mean the requirement is being dropped. A. Dhf Medical Device Template.
From www.cati.com
DHF Manager For Medical Device (REM) Computer Aided Technology Dhf Medical Device Template Is a collection of documents that describe the design and development activities of a medical device. A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Here’s what you need. Dhf Medical Device Template.
From templates.rjuuc.edu.np
Medical Device Design History File Template Dhf Medical Device Template Its purpose is to demonstrate that the device. In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Is a collection of documents that describe the design. Dhf Medical Device Template.
From www.youtube.com
How to Create Your DHF/TF & RMF for a Hardware or Software Medical Dhf Medical Device Template The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. But that doesn’t mean the requirement is being dropped. Its purpose is to demonstrate that the device. Explore the essential. Dhf Medical Device Template.
From www.greenlight.guru
3 Tips for Managing Your Medical Device Design History File Dhf Medical Device Template A guide to compiling a dhf for medical device development. A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. But that doesn’t mean the requirement is being dropped. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Explore. Dhf Medical Device Template.
From www.scilife.io
The 5 Medical Device Development Phases Scilife Dhf Medical Device Template The design history file (dhf): Is a collection of documents that describe the design and development activities of a medical device. A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: In. Dhf Medical Device Template.
From www.scilife.io
Differences between DHF, DMR, and DHR Scilife Dhf Medical Device Template A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. Its purpose is to demonstrate that the device. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: But that doesn’t mean the requirement is being dropped. The design history file (dhf):. Dhf Medical Device Template.
From sterlingmedicaldevices.com
Design History Files Everything You Should Know Dhf Medical Device Template Here’s what you need to know about the dhf and its ongoing importance in fda compliance. In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. A guide to compiling a dhf for medical device development. This white paper focuses on design control compliance for. Dhf Medical Device Template.
From www.qmdocs.com
Design History File (DHF) SOP QMDocs Quality Management System Templates Dhf Medical Device Template Explore the essential role of the design history file (dhf) in ensuring compliance, quality, and. In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. Is a collection of documents that describe the design and development activities of a medical device. The design history file. Dhf Medical Device Template.
From www.spkaa.com
The Trio of Documentation DHF, DMR, and DHR in Medical Device Dhf Medical Device Template Explore the essential role of the design history file (dhf) in ensuring compliance, quality, and. Its purpose is to demonstrate that the device. A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. But that doesn’t mean the requirement is being dropped. This white paper focuses on design control compliance. Dhf Medical Device Template.
From www.cati.com
DHF Manager For Medical Device (REM) Computer Aided Technology Dhf Medical Device Template This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Its purpose. Dhf Medical Device Template.
From www.qualitymeddev.com
Design History File for Medical Device An Overview Dhf Medical Device Template But that doesn’t mean the requirement is being dropped. Here’s what you need to know about the dhf and its ongoing importance in fda compliance. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Its purpose is to demonstrate that the device. The design history file (dhf): A guide to compiling. Dhf Medical Device Template.
From www.archimedic.com
Quality Managing the DHF for Medical Devices Dhf Medical Device Template The design history file (dhf): But that doesn’t mean the requirement is being dropped. In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. Its purpose is to demonstrate that the device. The fda’s new qmsr is dropping the reference to the dhf (design history. Dhf Medical Device Template.
From www.capgemini.com
A strategic approach towards DHF remediation of medical devices Capgemini Dhf Medical Device Template Here’s what you need to know about the dhf and its ongoing importance in fda compliance. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. A guide to compiling a dhf for medical device development. Its purpose is to demonstrate that the device. A dhf template could be created. Dhf Medical Device Template.
From www.greenlight.guru
Technical File vs. 510(k) vs. Design History File What Medical Device Dhf Medical Device Template Explore the essential role of the design history file (dhf) in ensuring compliance, quality, and. Is a collection of documents that describe the design and development activities of a medical device. Here’s what you need to know about the dhf and its ongoing importance in fda compliance. A dhf template could be created to follow a standardized design control process,. Dhf Medical Device Template.
From www.youtube.com
Use PTC Windchill to Manage FDA Medical Device Compliance YouTube Dhf Medical Device Template Explore the essential role of the design history file (dhf) in ensuring compliance, quality, and. In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. Here’s what you need to know about the dhf and its ongoing importance in fda compliance. A dhf template could. Dhf Medical Device Template.
From informacionpublica.svet.gob.gt
Assembling A Design History File (DHF) For Your Medical Dhf Medical Device Template A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. Its purpose is to demonstrate that the device. The design history file (dhf): Explore the essential role of the design history file (dhf) in ensuring compliance, quality, and. This white paper focuses on design control compliance for medical devices per. Dhf Medical Device Template.
From staging.youngvic.org
What is the difference of DHR DHF DMR and MDF Dhf Medical Device Template Explore the essential role of the design history file (dhf) in ensuring compliance, quality, and. Here’s what you need to know about the dhf and its ongoing importance in fda compliance. In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. A dhf template could. Dhf Medical Device Template.
From informacionpublica.svet.gob.gt
Assembling A Design History File (DHF) For Your Medical Dhf Medical Device Template Its purpose is to demonstrate that the device. A guide to compiling a dhf for medical device development. A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. But that doesn’t mean the requirement is being dropped. The design history file (dhf): This white paper focuses on design control compliance. Dhf Medical Device Template.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device Dhf Medical Device Template In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. The design history file (dhf): Here’s what you need to know about the dhf and its ongoing importance in fda compliance. Its purpose is to demonstrate that the device. This white paper focuses on design. Dhf Medical Device Template.
From www.qualio.com
Assembling a Design History File (DHF) for your medical device Dhf Medical Device Template In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. Is a collection of documents that describe the design and development activities of a medical device. Here’s what you need to know about the dhf and its ongoing importance in fda compliance. Its purpose is. Dhf Medical Device Template.
From medicaldeviceacademy.com
Design Control Medical Device Academy Dhf Medical Device Template A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. A guide to compiling a dhf for medical device development. In the realm of medical device development and regulatory compliance,. Dhf Medical Device Template.
From www.rcainc.com
DHF Design Controls Medical Device Consultants RCA® Dhf Medical Device Template The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Explore the essential role of the design history file (dhf) in ensuring compliance, quality, and. In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety.. Dhf Medical Device Template.
From www.greenlight.guru
How to Create Your DHF/TF & RMF for a Hardware or Software Medical Device Dhf Medical Device Template Its purpose is to demonstrate that the device. In the realm of medical device development and regulatory compliance, the design history file (dhf) plays a critical role in ensuring product quality and safety. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. But that doesn’t mean the requirement is. Dhf Medical Device Template.
From simplimedica.com
DHF Checklist Simplimedica Dhf Medical Device Template Here’s what you need to know about the dhf and its ongoing importance in fda compliance. A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. Its purpose is to demonstrate that the device. Is a collection of documents that describe the design and development activities of a medical device.. Dhf Medical Device Template.
From www.todaysmedicaldevelopments.com
Medical device industry Leveraging the digital thread Today's Dhf Medical Device Template Is a collection of documents that describe the design and development activities of a medical device. Its purpose is to demonstrate that the device. But that doesn’t mean the requirement is being dropped. Here’s what you need to know about the dhf and its ongoing importance in fda compliance. Explore the essential role of the design history file (dhf) in. Dhf Medical Device Template.
From prntbl.concejomunicipaldechinu.gov.co
Medical Device Verification And Validation Plan Template prntbl Dhf Medical Device Template The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. A dhf template could be created to follow a standardized design control process, but most manufacturers write a generic design. But that doesn’t mean the requirement is being dropped. Its purpose is to demonstrate that the device. The design history. Dhf Medical Device Template.