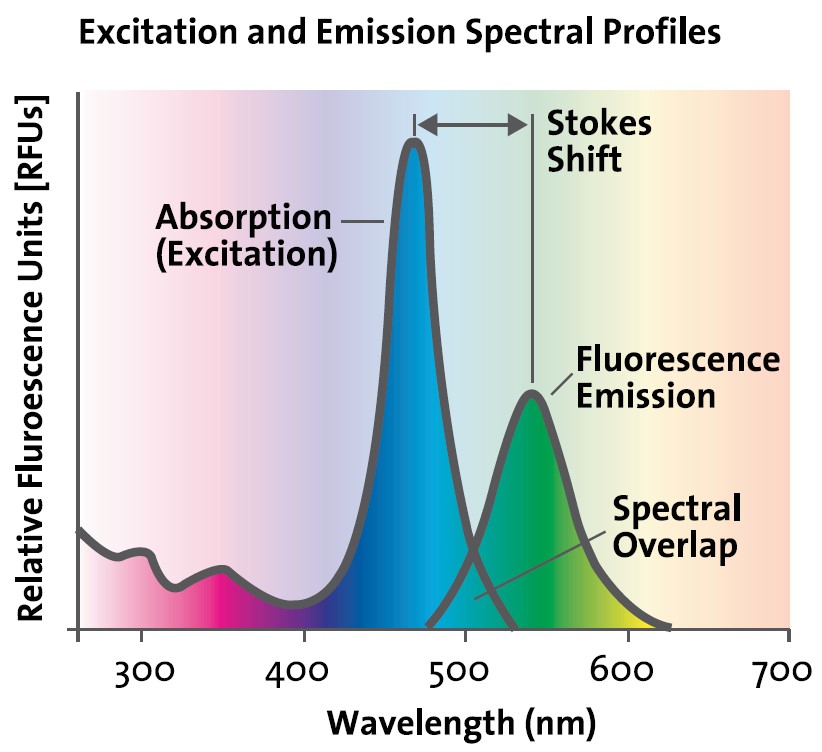
Fluorescence Spectroscopy Wavelength . at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual molecules or small. these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength, than the absorbed photon.
from www.tecan.com
as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength, than the absorbed photon. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual molecules or small.
How to develop an optimal fluorescence assay The Blog Tecan
Fluorescence Spectroscopy Wavelength these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual molecules or small. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength, than the absorbed photon.
From www.tecan.com
How to develop an optimal fluorescence assay The Blog Tecan Fluorescence Spectroscopy Wavelength a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Normalized absorption and fluorescence spectra of GFP and Cy3. The Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual molecules or small. a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. as the. Fluorescence Spectroscopy Wavelength.
From bitesizebio.com
Fluorescence Microscopy An Easy Guide for Biologists Fluorescence Spectroscopy Wavelength emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. . Fluorescence Spectroscopy Wavelength.
From www.pnas.org
Dynamic Stokes shift in green fluorescent protein variants PNAS Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. a plot. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Fluorescence spectra of GFP, E2Orange and mCherry. Shown are Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual molecules or small. as the. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
fluorescence spectroscopy with two wavelength Download Scientific Diagram Fluorescence Spectroscopy Wavelength a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength, than the absorbed. Fluorescence Spectroscopy Wavelength.
From www.xos.com
Monochromatic Wavelength Dispersive XRay Fluorescence XOS Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual molecules or small. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. . Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Fluorescence emission (blue and green), and excitation (red) spectra of Fluorescence Spectroscopy Wavelength as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength, than the absorbed photon. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual. Fluorescence Spectroscopy Wavelength.
From www.jasco-global.com
Principles of fluorescence spectroscopy (2) Features of fluorescence Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength, than the absorbed photon. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Fluorescence spectra of tyrosine at excitation wavelength of 200 nm (a Fluorescence Spectroscopy Wavelength a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual molecules or small. at wavelengths. Fluorescence Spectroscopy Wavelength.
From circuitengardo.z13.web.core.windows.net
Fluorescent Light Color Chart Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Normalised (A) absorption and (B) emission spectra of the fluorescent Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength, than the absorbed photon. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Fluorescence emission spectra (excitation wavelength = 365 nm) of the Fluorescence Spectroscopy Wavelength at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. a plot of emission against wavelength for any given excitation wavelength is known as the emission. Fluorescence Spectroscopy Wavelength.
From www.scientifica.uk.com
Fluorescence light sources A comparative guide Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual molecules or small. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. a plot. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
(A) Size dependent fluorescence spectra of quantum dots and (B Fluorescence Spectroscopy Wavelength at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength, than the absorbed photon. in an excitation spectrum, the light source is kept at a constant wavelength via. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
The absorption, fluorescence excitation and fluorescence spectra of ThT Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual molecules or small. as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength,. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Fluorescence spectra at various excitation wavelengths. (A Fluorescence Spectroscopy Wavelength as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength, than the absorbed photon. in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Absorption and fluorescence (excitation and emission) spectra of (3) in Fluorescence Spectroscopy Wavelength these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. . Fluorescence Spectroscopy Wavelength.
From www.jasco-global.com
Principles of fluorescence spectroscopy (2) Features of fluorescence Fluorescence Spectroscopy Wavelength these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. . Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Normalized excitation and fluorescence emission spectra of fluorescent Fluorescence Spectroscopy Wavelength a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Emission and excitation spectra of fluorescein showing the Stokes shift Fluorescence Spectroscopy Wavelength a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength, than the absorbed photon. these curves describe the likelihood that excitation and emission will occur as a function of wavelength. Fluorescence Spectroscopy Wavelength.
From www.horiba.com
What is ATEEM spectroscopy? HORIBA Fluorescence Spectroscopy Wavelength at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. using the. Fluorescence Spectroscopy Wavelength.
From www.scientifica.uk.com
Fluorescence light sources A comparative guide Fluorescence Spectroscopy Wavelength as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength, than the absorbed photon. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively. Fluorescence Spectroscopy Wavelength.
From ibsen.com
Fluorescense spectrum Ibsen Photonics Fluorescence Spectroscopy Wavelength at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual molecules or small. as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer. Fluorescence Spectroscopy Wavelength.
From jascoinc.com
Fluorescence Spectroscopy JASCO Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
The fluorescence spectrum of the fluorescence intensity at 535 nm at Fluorescence Spectroscopy Wavelength a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength, than the absorbed. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Basics of Fluorescence and FRET. ( a ) Visible light spectrum Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e.. Fluorescence Spectroscopy Wavelength.
From chem.libretexts.org
10.6 Photoluminescence Spectroscopy Chemistry LibreTexts Fluorescence Spectroscopy Wavelength these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Timeresolved fluorescence spectra and fluorescence decay curves at 77 Fluorescence Spectroscopy Wavelength emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. these curves describe. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
(a) Fluorescence emission spectra of carbon dots with excitation Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual molecules or small. a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum. these curves. Fluorescence Spectroscopy Wavelength.
From www.vrogue.co
A Fluorescence Emission Spectra And B Absorption Spec vrogue.co Fluorescence Spectroscopy Wavelength using the right excitation and emission wavelengths, fluorescence spectroscopy can be used to selectively detect individual molecules or small. in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength,. Fluorescence Spectroscopy Wavelength.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. a plot of emission against wavelength for any given excitation wavelength is known as the emission spectrum.. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Fluorescence spectrum of all compounds in DMSO. Wavelength = 350 nm (a Fluorescence Spectroscopy Wavelength at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. these curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the. a plot of emission against wavelength for any given excitation wavelength is known as the emission. Fluorescence Spectroscopy Wavelength.
From www.researchgate.net
Changes in the fluorescence spectrum and its integrated intensity Fluorescence Spectroscopy Wavelength in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator, and multiple. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. at wavelengths longer than 550 nanometers, the fluorophore molecules still absorb energy and fluoresce, but again in smaller proportions. these curves. Fluorescence Spectroscopy Wavelength.
From www.edinst.com
Reducing Fluorescence in Raman Spectroscopy Fluorescence Spectroscopy Wavelength as the excited molecule returns to ground state, emits a photon of lower energy, which corresponds to a longer wavelength, than the absorbed photon. emission spectrum ± excitation wavelength is kept constant and fluorescence intensity measured as function of wavelength, i.e. in an excitation spectrum, the light source is kept at a constant wavelength via the monochromator,. Fluorescence Spectroscopy Wavelength.