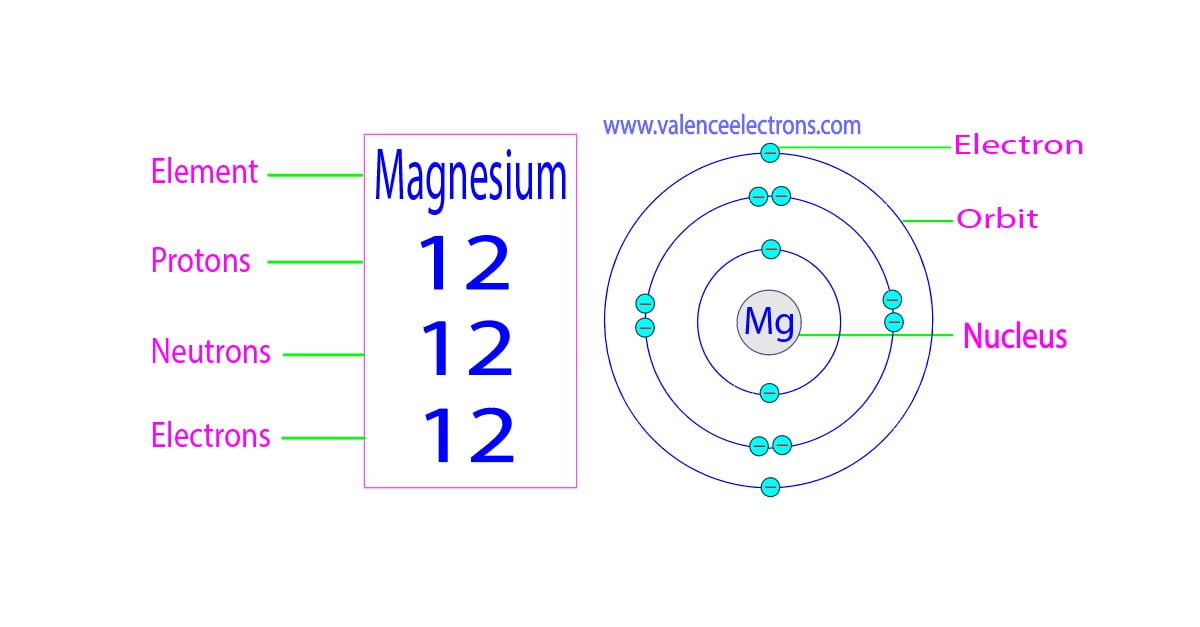
Magnesium The Charge . The most common charges are based on. The symbol for the ion is mg 2+ , and it is. Although both of these ions. 93 rows ionic charge: [1] you can see that the outermost orbit of magnesium has 2 electrons. the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg. During the chemical reaction, magnesium. the charge on an atom is related to its valence electrons or oxidation state. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).
from exoqkenlb.blob.core.windows.net
The most common charges are based on. During the chemical reaction, magnesium. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg. 93 rows ionic charge: the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg 2+ , and it is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.
Magnesium Has How Many Atomic Number at Rita Brown blog
Magnesium The Charge when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The most common charges are based on. The symbol for the ion is mg. the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); During the chemical reaction, magnesium. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg 2+ , and it is. [1] you can see that the outermost orbit of magnesium has 2 electrons. the charge on an atom is related to its valence electrons or oxidation state. 93 rows ionic charge: Although both of these ions. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).
From userdatabespangles.z14.web.core.windows.net
Lewis Diagram Of Magnesium Magnesium The Charge the charge on an atom is related to its valence electrons or oxidation state. The most common charges are based on. The symbol for the ion is mg. [1] you can see that the outermost orbit of magnesium has 2 electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an. Magnesium The Charge.
From nicolejardim.com
The Many Benefits of Magnesium for Your Health & Cycle Nicole Jardim Magnesium The Charge when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. 93 rows ionic charge: the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); The symbol for the ion is mg. During the chemical. Magnesium The Charge.
From www.numerade.com
SOLVED What happens when the compound Mgo is formed? (5 points) Oxygen Magnesium The Charge thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. 93 rows ionic charge: [1] you can see that the outermost orbit of magnesium has 2 electrons. the charge on an atom is related to its valence electrons or oxidation state. the charge is determined by. Magnesium The Charge.
From www.thoughtco.com
Magnesium Facts (Mg or Atomic Number 12) Magnesium The Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The symbol for the ion is mg. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The most common charges are based on. During the chemical reaction, magnesium. The. Magnesium The Charge.
From slideplayer.com
AS Chemistry Homework Periodicity ppt download Magnesium The Charge The symbol for the ion is mg 2+ , and it is. the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); During the chemical reaction, magnesium. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. thus, a magnesium. Magnesium The Charge.
From www.pinterest.com.au
Functions of magnesium in human body and sources in food outline Magnesium The Charge During the chemical reaction, magnesium. the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); Although both of these ions. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The symbol for the ion is. Magnesium The Charge.
From utedzz.blogspot.com
Periodic Table Magnesium Element Symbol Periodic Table Timeline Magnesium The Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The symbol for the ion is mg. Although both of these ions. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. the. Magnesium The Charge.
From cellhealthnews.com
Ten Forms of Magnesium Magnesium The Charge During the chemical reaction, magnesium. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The symbol for the ion is mg 2+ , and it is. thus, a magnesium atom will form a cation with two fewer electrons than protons and a. Magnesium The Charge.
From userdatatactilists.z14.web.core.windows.net
Magnesium Energy Level Diagram Magnesium The Charge The most common charges are based on. the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. [1] you can see that the outermost orbit of magnesium has 2 electrons.. Magnesium The Charge.
From www.cloudizsexy.com
Magnesium Atomic Number Atomic Mass Density Of Magnesium Free Hot Magnesium The Charge The symbol for the ion is mg. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. thus, a magnesium atom will form. Magnesium The Charge.
From www.youtube.com
"Magnesium The Key Nutrient for a Healthier You Here's How to Get It Magnesium The Charge the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg 2+ , and it is. when an ionic compound is formed from magnesium. Magnesium The Charge.
From montessorimuddle.org
subatomic particles Montessori Muddle Magnesium The Charge thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The most common charges are based on. The symbol for the ion is mg 2+ , and it is. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. When. Magnesium The Charge.
From slideplayer.com
“My name is Bond, Ionic Bond; taken, not shared!” ppt download Magnesium The Charge when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. When the atom loses or gains one or more electrons, the electric charge is. Magnesium The Charge.
From www.nagwa.com
Question Video Calculating the Relative Formula Mass of Magnesium Magnesium The Charge 93 rows ionic charge: The most common charges are based on. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The symbol. Magnesium The Charge.
From www.youtube.com
magnesium ion charge YouTube Magnesium The Charge thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen. Magnesium The Charge.
From wirelistsightsmen.z21.web.core.windows.net
Lewis Dot Diagram For Magnesium Oxide Magnesium The Charge During the chemical reaction, magnesium. The most common charges are based on. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. [1] you can see that the outermost orbit of magnesium has 2 electrons. The symbol for the ion is mg 2+ , and it is. 93. Magnesium The Charge.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.40 Draw DotandCross Diagrams to Show the Magnesium The Charge Although both of these ions. the charge on an atom is related to its valence electrons or oxidation state. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. the charge is determined by taking 17 (the number of protons) and subtracting. Magnesium The Charge.
From brainly.in
The element magnesium has 2 valence electrons.What is the most likely Magnesium The Charge [1] you can see that the outermost orbit of magnesium has 2 electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); During the chemical reaction, magnesium. thus,. Magnesium The Charge.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium The Charge thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. the charge on an atom is related to its valence electrons or oxidation state. The most common charges are based on. Although both of these ions. the charge is determined by taking 17 (the number of protons) and. Magnesium The Charge.
From www.shutterstock.com
Magnesium Atom. Diagram Representation Of The Element Magnesium Magnesium The Charge The symbol for the ion is mg 2+ , and it is. the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. the charge on an atom is related to. Magnesium The Charge.
From alexamood.com
The health importance of Magnesium Alexamood Magnesium The Charge thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The most common charges are based on. [1] you can see that the outermost orbit of magnesium has 2 electrons. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and. Magnesium The Charge.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model Magnesium The Charge Although both of these ions. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. [1] you can see that the outermost orbit of magnesium has 2 electrons. During the chemical reaction, magnesium. the charge on an atom is related to its. Magnesium The Charge.
From www.britannica.com
Magnesium Description, Properties, & Compounds Britannica Magnesium The Charge the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); During the chemical reaction, magnesium. [1] you can see that the outermost orbit of magnesium has 2 electrons. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. when. Magnesium The Charge.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Magnesium The Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: Although both of these ions. the charge on an atom is related to its valence electrons or oxidation state. when an ionic compound is formed from magnesium and oxygen, the magnesium ion has. Magnesium The Charge.
From www.youtube.com
How to Find the Ionic Charge for Magnesium (Mg) YouTube Magnesium The Charge The symbol for the ion is mg. The symbol for the ion is mg 2+ , and it is. 93 rows ionic charge: During the chemical reaction, magnesium. the charge on an atom is related to its valence electrons or oxidation state. [1] you can see that the outermost orbit of magnesium has 2 electrons. The most. Magnesium The Charge.
From brainly.com
What is the charge on a magnesium ion? How do they get that charge Magnesium The Charge The symbol for the ion is mg. The most common charges are based on. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. the charge on an atom is related to its valence electrons or oxidation state. When the atom loses or gains one or more electrons, the. Magnesium The Charge.
From mychirodoc.co.za
Magnesium and the function of various formulations My Chiro Doc Magnesium The Charge the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. Although both of these ions. The symbol for the ion is mg 2+ , and it is. When the atom loses. Magnesium The Charge.
From www.youtube.com
What is magnesium’s charge? YouTube Magnesium The Charge thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the. Magnesium The Charge.
From www.myxxgirl.com
Magnesium Element Tile Periodic Table Sticker By Sciencenotes My XXX Magnesium The Charge thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. During the chemical reaction, magnesium. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. the charge on an atom is related to its valence electrons or oxidation state.. Magnesium The Charge.
From slidetodoc.com
Metal ions Nonmetal ions Positive ion Gain electrons Magnesium The Charge the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); 93 rows ionic charge: The symbol for the ion is mg 2+ , and it is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). when an ionic. Magnesium The Charge.
From www.toppr.com
When magnesium makes an ionic bond with oxygen it loses two electrons Magnesium The Charge [1] you can see that the outermost orbit of magnesium has 2 electrons. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. 93 rows ionic charge: The symbol for the ion is mg. When the atom loses or gains one or more electrons, the electric charge is. Magnesium The Charge.
From www.chegg.com
Solved Predict the charge that the ion formed from magnesium Magnesium The Charge Although both of these ions. the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); [1] you can see that the outermost orbit of magnesium has 2 electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The symbol. Magnesium The Charge.
From detoxthebodymcs.com
Magnesium Benefits Chart — Detox your Body & Mind Magnesium The Charge The symbol for the ion is mg. the charge on an atom is related to its valence electrons or oxidation state. the charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); Although both of these ions. when an ionic compound is formed from magnesium and oxygen, the magnesium ion. Magnesium The Charge.
From www.dreamstime.com
Magnesium Mg Chemical Element. Magnesium Sign with Atomic Number Magnesium The Charge thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg 2+ , and it is. Although both of these ions. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for. Magnesium The Charge.
From exoqkenlb.blob.core.windows.net
Magnesium Has How Many Atomic Number at Rita Brown blog Magnesium The Charge The most common charges are based on. The symbol for the ion is mg. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.. Magnesium The Charge.