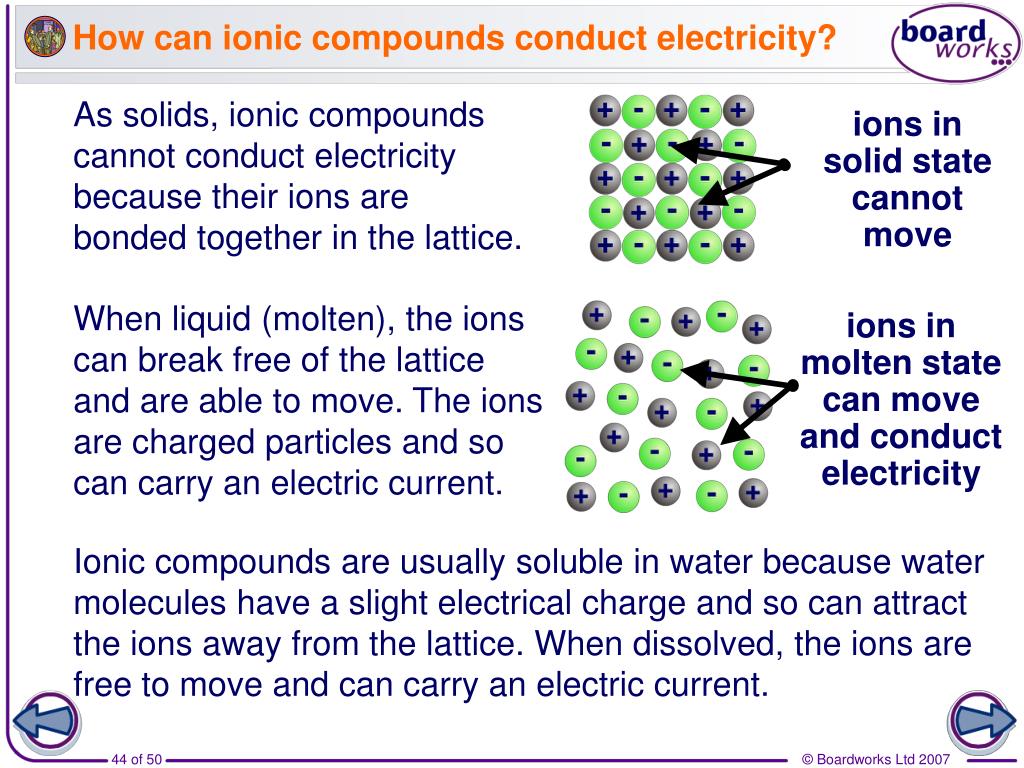
Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water . Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry a current. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. Learn the difference between ionic and covalent bonds, and how dissociation and electrodes affect the conductivity of ionic compounds. The strong bonds between their oppositely charged ions lock them into place in. This is because the ions are free to. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Ionic compounds are good conductors of electricity when they are dissolved in water or melted, because their ions are free to move. Ionic compounds conduct an electric current when melted or dissolved in water. Solid ionic compounds are poor conductors of electricity.
from ceopyitw.blob.core.windows.net
This is because the ions are free to. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. Solid ionic compounds are poor conductors of electricity. The strong bonds between their oppositely charged ions lock them into place in. Ionic compounds conduct an electric current when melted or dissolved in water. Ionic compounds are good conductors of electricity when they are dissolved in water or melted, because their ions are free to move. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry a current. Learn the difference between ionic and covalent bonds, and how dissociation and electrodes affect the conductivity of ionic compounds.
What Compounds When Dissolved In Water Conduct Electricity at Jon
Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds are good conductors of electricity when they are dissolved in water or melted, because their ions are free to move. Learn the difference between ionic and covalent bonds, and how dissociation and electrodes affect the conductivity of ionic compounds. Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry a current. Ionic compounds conduct an electric current when melted or dissolved in water. The strong bonds between their oppositely charged ions lock them into place in. This is because the ions are free to. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. Ionic compounds are good conductors of electricity when they are dissolved in water or melted, because their ions are free to move. Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Solid ionic compounds are poor conductors of electricity.
From www.slideshare.net
Chem matters ch6_ionic_bond Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water This is because the ions are free to. Ionic compounds are good conductors of electricity when they are dissolved in water or melted, because their ions are free to move. Ionic compounds conduct an electric current when melted or dissolved in water. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Ch. 7 LT1 Ionic Bonding and Metals ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds conduct an electric current when melted or dissolved in water. Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry a current. Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Learn the difference between ionic and covalent bonds, and how. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From ec.bertabulle.com
How Do Ions Conduct Electricity Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. This is because the ions are free to. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. Learn the difference between ionic and covalent bonds, and how dissociation and. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
John A. Schreifels Chemistry 211notes 1 CHAPTER 4 Chemical Reactions Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Ionic compounds are good conductors of electricity when they are dissolved in water or melted, because their ions are free to move. The strong bonds between their oppositely charged ions lock them into place in. Ionic compounds are good. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Chapter 4 Chemical Quantities and ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water This is because the ions are free to. Ionic compounds conduct an electric current when melted or dissolved in water. Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Learn the difference between ionic and covalent bonds, and how dissociation and electrodes affect the conductivity of ionic compounds. Ionic compounds dissociate into charged. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Chemistry Ionic Compounds. ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds conduct an electric current when melted or dissolved in water. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. Ionic compounds are good conductors of electricity when. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
CHEMICAL BONDING Cocaine Chemistry I Chapter 8 ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry a current. This is because the ions are free to. Ionic compounds can conduct electricity when they are dissolved. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Bonding Year 11 Chemistry. ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. Solid ionic compounds are poor conductors of electricity. Ionic compounds dissociate into charged ions when dissolved in water, which can. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From ceopyitw.blob.core.windows.net
What Compounds When Dissolved In Water Conduct Electricity at Jon Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water The strong bonds between their oppositely charged ions lock them into place in. Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry a current. Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Learn the difference between ionic and covalent bonds, and. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. The strong bonds between their oppositely charged ions lock them into place in. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Learn the difference between ionic and covalent bonds, and how. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From www.slideserve.com
PPT Chapter 7 Properties of Ionic Covalent and Metal Materials Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry a current. Ionic compounds conduct an electric current when melted or dissolved in water. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. The strong bonds between their oppositely charged. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slidetodoc.com
MYP Chemistry Ionic Bonding and Ionic Compounds International Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. The strong bonds between their oppositely charged ions lock them into place in. Ionic compounds conduct an electric current when melted or dissolved in water. Learn the difference between ionic and covalent bonds, and how dissociation and electrodes affect. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Chapter 7 Properties of Ionic Covalent and Metal Materials ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds are good conductors of electricity when they are dissolved in water or melted, because their ions are free to move. Learn the difference between ionic and covalent bonds, and how dissociation and electrodes affect the conductivity of ionic compounds. Solid ionic compounds are poor conductors of electricity. Ionic compounds dissociate into charged ions when dissolved in water, which. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From chem.libretexts.org
7.5 Aqueous Solutions and Solubility Compounds Dissolved in Water Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry a current. This is because the ions are free to. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. The strong bonds between their oppositely charged ions lock. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Learn the difference between ionic and covalent bonds, and how dissociation and electrodes affect the conductivity of ionic compounds. Ionic compounds are good. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Unit 2 Bonding. ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. This is because the ions are free to. Learn the difference between ionic and covalent bonds, and how dissociation and electrodes affect the conductivity of ionic compounds. Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From www.slideserve.com
PPT IONIc bonds and ionic compounds PowerPoint Presentation, free Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water The strong bonds between their oppositely charged ions lock them into place in. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. Learn the difference between ionic and covalent bonds, and how dissociation and electrodes affect the conductivity of ionic compounds. Ionic compounds are not good at conducting electricity in. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water The strong bonds between their oppositely charged ions lock them into place in. Solid ionic compounds are poor conductors of electricity. Ionic compounds are good conductors of electricity when they are dissolved in water or melted, because their ions are free to move. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Additional Chapter 5 information ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water This is because the ions are free to. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Ionic compounds are good conductors of electricity when they are dissolved in. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry a current. Ionic compounds are good conductors of electricity when they are dissolved in water or melted, because their ions are free to move.. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From www.teachoo.com
The table shown below gives information about four Chemistry MCQ Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water The strong bonds between their oppositely charged ions lock them into place in. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. This is because the ions are free to. Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From atlas-scientific.com
How Do Ions Increase Conductivity? Atlas Scientific Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water This is because the ions are free to. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. The strong bonds between their oppositely charged ions lock them into place in. This is because the ions are free to. Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Chemical Bonding Lesson 1 Ionic Bonds & Compounds. ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry a current. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Ionic. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Solid ionic compounds are poor conductors of electricity. Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry a current. The strong bonds between their oppositely charged ions lock them into place in. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From www.numerade.com
SOLVED 'Why do ionic compounds make good conductors when dissolved in Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Ionic compounds conduct an electric current when melted or dissolved in water. Solid ionic compounds are poor conductors of electricity. The strong bonds between their. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Introduction to Chemical Reactions ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water The strong bonds between their oppositely charged ions lock them into place in. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. Learn the difference between ionic and covalent bonds, and how dissociation and electrodes affect the conductivity of ionic compounds. Ionic compounds can conduct electricity when they are dissolved. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds are good conductors of electricity when they are dissolved in water or melted, because their ions are free to move. Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Solid ionic compounds are poor conductors of electricity. The strong bonds between their oppositely charged ions lock them into place in. Learn. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From ec.bertabulle.com
How Do Ions Conduct Electricity Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. This is because the ions are free to. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Ionic compounds. ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water This is because the ions are free to. The strong bonds between their oppositely charged ions lock them into place in. Ionic compounds are good conductors of electricity when they are dissolved in water or melted, because their ions are free to move. Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Ionic. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Chemical Bonds. ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds dissociate into charged ions when dissolved in water, which can move freely in the electric field and carry a current. The strong bonds between their oppositely charged ions lock them into place in. Ionic compounds are good conductors of electricity when they are dissolved in water or melted, because their ions are free to move. Solid ionic compounds. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Ionic Compounds. ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds are good conductors of electricity when they are dissolved in water or melted, because their ions are free to move. Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Solid ionic compounds are poor conductors of electricity. Ionic compounds dissociate into charged ions when dissolved in water, which can move freely. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Intramolecular Forces Intermolecular Forces ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds can conduct electricity when they are dissolved in water or when they are melted. Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. The strong bonds between their oppositely charged ions lock them into place in. Ionic compounds are good conductors of electricity when they are dissolved in. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Chemical BONDING. ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water The strong bonds between their oppositely charged ions lock them into place in. Ionic compounds conduct an electric current when melted or dissolved in water. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Ionic compounds dissociate into charged ions when dissolved in water, which can move freely. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.
From slideplayer.com
Chem 1 Chapter 8 Ionic Bonding ppt download Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water Ionic compounds are good conductors of electricity when dissolved in water or melted, because their ions can move freely. Solid ionic compounds are poor conductors of electricity. Ionic compounds are not good at conducting electricity in the solid state, but they do conduct electricity when dissolved in water. Ionic compounds can conduct electricity when they are dissolved in water or. Are Ionic Compounds Good Conductors Of Electricity When Dissolved In Water.