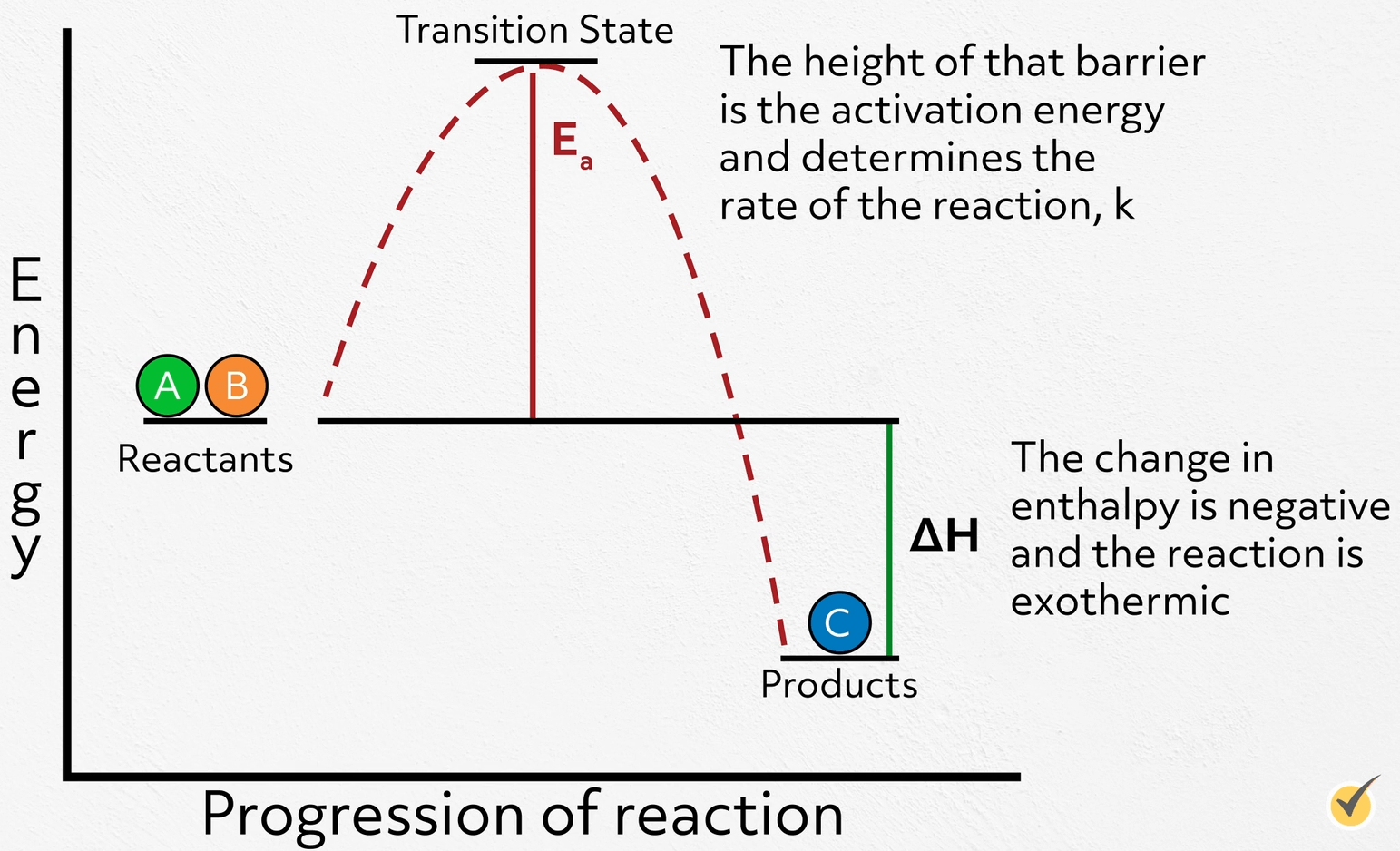
Catalyst Reaction Of Exothermic . Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. An exothermic reaction causes the surroundings to heat up. Because the surroundings is gaining heat from. The catalyst would act in the same way for an exothermic reaction. (2009) observed an exothermic reaction of. Explain the function of a catalyst in terms of reaction mechanisms and. A chemical reaction is said to be exothermic when it releases energy in the form of heat. For example, the burning of wood releases heat. The effect of a catalyst on the activation energy of an endothermic reaction. Describe three ways to speed up a reaction. Methanol synthesis runs near equilibrium, since it is an exothermic reaction, and yield diminishes at high temperatures (see figure 7). The system (reaction) releases heat to the surroundings as the reactants transform into products. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. A chemical reaction is exothermic if heat is released by the system into the surroundings. By the end of this section, you will be able to:
from ar.inspiredpencil.com
A chemical reaction is exothermic if heat is released by the system into the surroundings. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. For example, the burning of wood releases heat. (2009) observed an exothermic reaction of. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Explain the function of a catalyst in terms of reaction mechanisms and. By the end of this section, you will be able to: Because the surroundings is gaining heat from. Methanol synthesis runs near equilibrium, since it is an exothermic reaction, and yield diminishes at high temperatures (see figure 7). An exothermic reaction causes the surroundings to heat up.
Exothermic Reaction With Catalyst
Catalyst Reaction Of Exothermic For example, the burning of wood releases heat. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. An exothermic reaction causes the surroundings to heat up. The effect of a catalyst on the activation energy of an endothermic reaction. Explain the function of a catalyst in terms of reaction mechanisms and. For example, the burning of wood releases heat. By the end of this section, you will be able to: Because the surroundings is gaining heat from. Describe three ways to speed up a reaction. Methanol synthesis runs near equilibrium, since it is an exothermic reaction, and yield diminishes at high temperatures (see figure 7). The system (reaction) releases heat to the surroundings as the reactants transform into products. (2009) observed an exothermic reaction of. A chemical reaction is said to be exothermic when it releases energy in the form of heat. The catalyst would act in the same way for an exothermic reaction. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. A chemical reaction is exothermic if heat is released by the system into the surroundings.
From www.revisechemistry.uk
Energetics OCR Gateway C3 revisechemistry.uk Catalyst Reaction Of Exothermic By the end of this section, you will be able to: The system (reaction) releases heat to the surroundings as the reactants transform into products. (2009) observed an exothermic reaction of. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have. Catalyst Reaction Of Exothermic.
From keystagewiki.com
Exothermic Key Stage Wiki Catalyst Reaction Of Exothermic (2009) observed an exothermic reaction of. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. Because the surroundings is gaining heat from. Describe three ways to speed up a reaction. The catalyst would act in the same way for an exothermic reaction. For example, the burning of wood releases heat. Explain the function of a catalyst in. Catalyst Reaction Of Exothermic.
From ar.inspiredpencil.com
Exothermic Reaction With Catalyst Catalyst Reaction Of Exothermic For example, the burning of wood releases heat. (2009) observed an exothermic reaction of. The system (reaction) releases heat to the surroundings as the reactants transform into products. A chemical reaction is exothermic if heat is released by the system into the surroundings. A chemical reaction is said to be exothermic when it releases energy in the form of heat.. Catalyst Reaction Of Exothermic.
From 2012books.lardbucket.org
Catalysis Catalyst Reaction Of Exothermic Explain the function of a catalyst in terms of reaction mechanisms and. For example, the burning of wood releases heat. An exothermic reaction causes the surroundings to heat up. A chemical reaction is said to be exothermic when it releases energy in the form of heat. The effect of a catalyst on the activation energy of an endothermic reaction. Differentiate. Catalyst Reaction Of Exothermic.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Catalyst Reaction Of Exothermic A chemical reaction is said to be exothermic when it releases energy in the form of heat. An exothermic reaction causes the surroundings to heat up. Because the surroundings is gaining heat from. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. Catalyst Reaction Of Exothermic.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyst Reaction Of Exothermic For example, the burning of wood releases heat. The effect of a catalyst on the activation energy of an endothermic reaction. Describe three ways to speed up a reaction. Methanol synthesis runs near equilibrium, since it is an exothermic reaction, and yield diminishes at high temperatures (see figure 7). Fluctuations in activity, hot zones in reactors are characteristic of exothermic. Catalyst Reaction Of Exothermic.
From www.slideserve.com
PPT Higher Chemistry Unit 1(b) Potential energy diagrams PowerPoint Catalyst Reaction Of Exothermic Methanol synthesis runs near equilibrium, since it is an exothermic reaction, and yield diminishes at high temperatures (see figure 7). Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. For example, the burning of wood releases heat. The effect of a catalyst on the activation energy of an endothermic reaction. Differentiate between reversible exothermic, irreversible exothermic, and. Catalyst Reaction Of Exothermic.
From alternatordiagram.blogspot.com
Exothermic Reaction Energy Diagram alternator Catalyst Reaction Of Exothermic Describe three ways to speed up a reaction. A chemical reaction is exothermic if heat is released by the system into the surroundings. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. The catalyst would act in the same way for an exothermic reaction. The system (reaction) releases heat to the surroundings as the reactants transform into. Catalyst Reaction Of Exothermic.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Reaction Of Exothermic A chemical reaction is said to be exothermic when it releases energy in the form of heat. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Explain the function of a catalyst in terms of reaction mechanisms. Catalyst Reaction Of Exothermic.
From ar.inspiredpencil.com
Exothermic Reaction With Catalyst Catalyst Reaction Of Exothermic Methanol synthesis runs near equilibrium, since it is an exothermic reaction, and yield diminishes at high temperatures (see figure 7). An exothermic reaction causes the surroundings to heat up. The catalyst would act in the same way for an exothermic reaction. A chemical reaction is said to be exothermic when it releases energy in the form of heat. Describe three. Catalyst Reaction Of Exothermic.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Catalyst Reaction Of Exothermic Methanol synthesis runs near equilibrium, since it is an exothermic reaction, and yield diminishes at high temperatures (see figure 7). Describe three ways to speed up a reaction. A chemical reaction is exothermic if heat is released by the system into the surroundings. The catalyst would act in the same way for an exothermic reaction. The effect of a catalyst. Catalyst Reaction Of Exothermic.
From slideplayer.com
Catalysts, Endothermic vs. Exothermic Reactions ppt download Catalyst Reaction Of Exothermic The catalyst would act in the same way for an exothermic reaction. The system (reaction) releases heat to the surroundings as the reactants transform into products. An exothermic reaction causes the surroundings to heat up. (2009) observed an exothermic reaction of. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis. Catalyst Reaction Of Exothermic.
From mungfali.com
Exothermic Reaction Energy Graph Catalyst Reaction Of Exothermic By the end of this section, you will be able to: For example, the burning of wood releases heat. The catalyst would act in the same way for an exothermic reaction. The effect of a catalyst on the activation energy of an endothermic reaction. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. (2009) observed an exothermic. Catalyst Reaction Of Exothermic.
From www.researchgate.net
Temperature gradient due to exothermic reaction of catalyst. Download Catalyst Reaction Of Exothermic Because the surroundings is gaining heat from. By the end of this section, you will be able to: Describe three ways to speed up a reaction. Methanol synthesis runs near equilibrium, since it is an exothermic reaction, and yield diminishes at high temperatures (see figure 7). An exothermic reaction causes the surroundings to heat up. The system (reaction) releases heat. Catalyst Reaction Of Exothermic.
From nonsibihighschool.org
Section 184 Catalysis Catalyst Reaction Of Exothermic Explain the function of a catalyst in terms of reaction mechanisms and. The system (reaction) releases heat to the surroundings as the reactants transform into products. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. For example,. Catalyst Reaction Of Exothermic.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Reaction Of Exothermic Methanol synthesis runs near equilibrium, since it is an exothermic reaction, and yield diminishes at high temperatures (see figure 7). Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. The effect of a catalyst on the activation. Catalyst Reaction Of Exothermic.
From mungfali.com
Exothermic Reaction Energy Profile Diagram Catalyst Reaction Of Exothermic (2009) observed an exothermic reaction of. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. Explain the function of a catalyst in terms of reaction mechanisms and. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on. Catalyst Reaction Of Exothermic.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Catalyst Reaction Of Exothermic A chemical reaction is said to be exothermic when it releases energy in the form of heat. For example, the burning of wood releases heat. Methanol synthesis runs near equilibrium, since it is an exothermic reaction, and yield diminishes at high temperatures (see figure 7). Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. Catalyst Reaction Of Exothermic.
From ar.inspiredpencil.com
Exothermic Reaction With Catalyst Catalyst Reaction Of Exothermic A chemical reaction is said to be exothermic when it releases energy in the form of heat. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. For example, the burning of wood releases heat. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and. Catalyst Reaction Of Exothermic.
From eduinput.com
Exothermic ReactionsCharacteristics, Identification, and Examples Catalyst Reaction Of Exothermic The catalyst would act in the same way for an exothermic reaction. A chemical reaction is exothermic if heat is released by the system into the surroundings. (2009) observed an exothermic reaction of. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. Catalyst Reaction Of Exothermic.
From www.chemistrylearner.com
Exothermic Reaction Definition, Equation, and Examples Catalyst Reaction Of Exothermic Explain the function of a catalyst in terms of reaction mechanisms and. For example, the burning of wood releases heat. An exothermic reaction causes the surroundings to heat up. Methanol synthesis runs near equilibrium, since it is an exothermic reaction, and yield diminishes at high temperatures (see figure 7). By the end of this section, you will be able to:. Catalyst Reaction Of Exothermic.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID3558810 Catalyst Reaction Of Exothermic (2009) observed an exothermic reaction of. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. An exothermic reaction causes the surroundings to heat up. The system (reaction) releases heat to the surroundings as the reactants transform into. Catalyst Reaction Of Exothermic.
From mavink.com
Exothermic Chemical Reaction Examples Catalyst Reaction Of Exothermic The effect of a catalyst on the activation energy of an endothermic reaction. A chemical reaction is exothermic if heat is released by the system into the surroundings. Because the surroundings is gaining heat from. Explain the function of a catalyst in terms of reaction mechanisms and. For example, the burning of wood releases heat. An exothermic reaction causes the. Catalyst Reaction Of Exothermic.
From www.worksheetsplanet.com
What is an Exothermic Reaction Definition and Example Catalyst Reaction Of Exothermic Describe three ways to speed up a reaction. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. By the end of this section, you will. Catalyst Reaction Of Exothermic.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Exothermic Catalyst Reaction Of Exothermic The catalyst would act in the same way for an exothermic reaction. Methanol synthesis runs near equilibrium, since it is an exothermic reaction, and yield diminishes at high temperatures (see figure 7). Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. For example, the burning of wood releases heat. A chemical reaction is said to be exothermic. Catalyst Reaction Of Exothermic.
From ar.inspiredpencil.com
Exothermic Reaction With Catalyst Catalyst Reaction Of Exothermic By the end of this section, you will be able to: The effect of a catalyst on the activation energy of an endothermic reaction. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. For example, the burning of wood releases heat. An exothermic reaction causes the surroundings to heat up. A chemical reaction is exothermic if heat. Catalyst Reaction Of Exothermic.
From manualpartsynaxis123.z13.web.core.windows.net
Exothermic And Endothermic Energy Diagrams Catalyst Reaction Of Exothermic An exothermic reaction causes the surroundings to heat up. Describe three ways to speed up a reaction. A chemical reaction is exothermic if heat is released by the system into the surroundings. For example, the burning of wood releases heat. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels,. Catalyst Reaction Of Exothermic.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Catalyst Reaction Of Exothermic A chemical reaction is said to be exothermic when it releases energy in the form of heat. The system (reaction) releases heat to the surroundings as the reactants transform into products. Because the surroundings is gaining heat from. An exothermic reaction causes the surroundings to heat up. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. A. Catalyst Reaction Of Exothermic.
From ar.inspiredpencil.com
Exothermic Reaction With Catalyst Catalyst Reaction Of Exothermic Because the surroundings is gaining heat from. By the end of this section, you will be able to: A chemical reaction is exothermic if heat is released by the system into the surroundings. (2009) observed an exothermic reaction of. A chemical reaction is said to be exothermic when it releases energy in the form of heat. For example, the burning. Catalyst Reaction Of Exothermic.
From www.chemistrylearner.com
Exothermic Reaction Definition, Equation, and Examples Catalyst Reaction Of Exothermic Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. A chemical reaction is exothermic if heat is released by the system into the surroundings. (2009) observed an exothermic reaction of. Because the surroundings is gaining heat from. For example, the burning of wood releases heat. The catalyst would act in the same way for an exothermic reaction.. Catalyst Reaction Of Exothermic.
From www.sliderbase.com
Endothermic Reaction w/Catalyst Catalyst Reaction Of Exothermic The system (reaction) releases heat to the surroundings as the reactants transform into products. Explain the function of a catalyst in terms of reaction mechanisms and. The catalyst would act in the same way for an exothermic reaction. The effect of a catalyst on the activation energy of an endothermic reaction. An exothermic reaction causes the surroundings to heat up.. Catalyst Reaction Of Exothermic.
From mungfali.com
Exothermic Reaction Energy Profile Diagram Catalyst Reaction Of Exothermic The catalyst would act in the same way for an exothermic reaction. Fluctuations in activity, hot zones in reactors are characteristic of exothermic reactions. Explain the function of a catalyst in terms of reaction mechanisms and. For example, the burning of wood releases heat. The system (reaction) releases heat to the surroundings as the reactants transform into products. Describe three. Catalyst Reaction Of Exothermic.
From byjus.com
24. What is the Minimum activation energy required for endothermic and Catalyst Reaction Of Exothermic By the end of this section, you will be able to: Describe three ways to speed up a reaction. (2009) observed an exothermic reaction of. A chemical reaction is said to be exothermic when it releases energy in the form of heat. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include. Catalyst Reaction Of Exothermic.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Reaction Of Exothermic Describe three ways to speed up a reaction. The effect of a catalyst on the activation energy of an endothermic reaction. By the end of this section, you will be able to: Because the surroundings is gaining heat from. A chemical reaction is exothermic if heat is released by the system into the surroundings. The system (reaction) releases heat to. Catalyst Reaction Of Exothermic.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalyst Reaction Of Exothermic A chemical reaction is said to be exothermic when it releases energy in the form of heat. The effect of a catalyst on the activation energy of an endothermic reaction. For example, the burning of wood releases heat. A chemical reaction is exothermic if heat is released by the system into the surroundings. By the end of this section, you. Catalyst Reaction Of Exothermic.