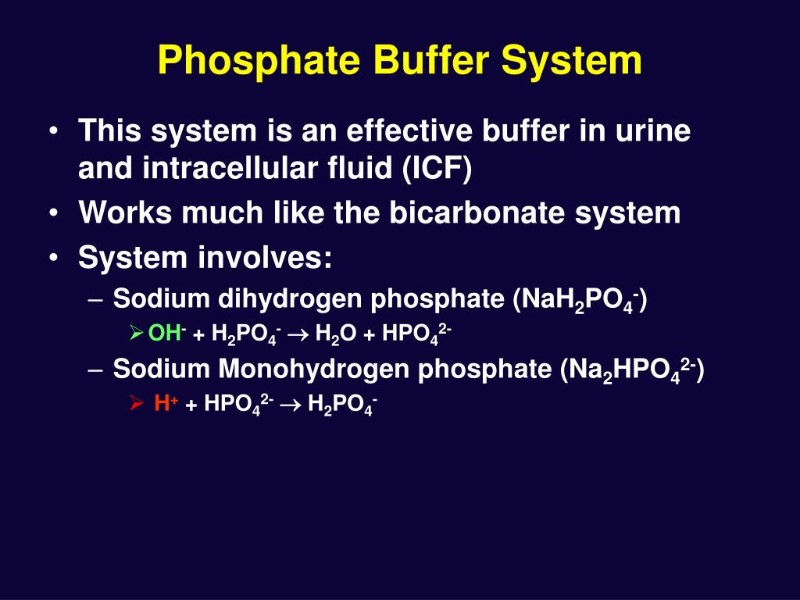
Phosphate Buffer Reaction . Recipe can be automatically scaled by entering desired final volume. For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction of the buffer components when a strong acid and a strong base is. Add 11.555 g of sodium phosphate dibasic to the solution. Phosphate acts best as a buffer in the reqions of ph near its three pk's: Pk 1 = 2.12, pk 2 = 7.21; How do you prepare your phosphate buffer? Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. And pk 3 = 12.44. Because phosphoric acid has multiple. Add 2.231 g of sodium dihydrogen phosphate to the solution. Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. A phosphate buffer solution is a handy buffer to have around, especially for biological applications.
from operaresidences.com.au
Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction of the buffer components when a strong acid and a strong base is. Add 11.555 g of sodium phosphate dibasic to the solution. Add 2.231 g of sodium dihydrogen phosphate to the solution. Phosphate acts best as a buffer in the reqions of ph near its three pk's: Pk 1 = 2.12, pk 2 = 7.21; Because phosphoric acid has multiple. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Recipe can be automatically scaled by entering desired final volume.
Describe the role of the phosphate buffer system? Opera Residences
Phosphate Buffer Reaction Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Because phosphoric acid has multiple. Pk 1 = 2.12, pk 2 = 7.21; Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. How do you prepare your phosphate buffer? Recipe can be automatically scaled by entering desired final volume. Add 11.555 g of sodium phosphate dibasic to the solution. Phosphate acts best as a buffer in the reqions of ph near its three pk's: Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction of the buffer components when a strong acid and a strong base is. Add 2.231 g of sodium dihydrogen phosphate to the solution. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. And pk 3 = 12.44.
From chem.libretexts.org
10.2 Phosphorylation reactions kinase enzymes Chemistry LibreTexts Phosphate Buffer Reaction Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Recipe can be automatically scaled by entering desired final volume. And pk 3 = 12.44. Pk 1 = 2.12, pk 2 = 7.21; Add 2.231 g of sodium dihydrogen phosphate to the solution. Add 11.555 g of sodium phosphate dibasic to the solution. Because phosphoric acid has multiple. Phosphate buffer. Phosphate Buffer Reaction.
From www.cephamls.com
Phosphate Buffer, pH 8.0 [0.5 M] Cepham Life Sciences Research Products Phosphate Buffer Reaction Add 11.555 g of sodium phosphate dibasic to the solution. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. Pk 1 = 2.12, pk 2 = 7.21; Phosphate acts best as a buffer in the reqions of ph near its three pk's: Because. Phosphate Buffer Reaction.
From fdneurotech.com
0.1 M Phosphate Buffer, pH 7.4 FD Neuro Technologies, Inc. Phosphate Buffer Reaction How do you prepare your phosphate buffer? Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Add 2.231 g of sodium dihydrogen phosphate to the solution. Pk 1 = 2.12, pk 2 = 7.21; Because phosphoric acid has multiple. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Gomori buffers, the most commonly used phosphate buffers, consist of. Phosphate Buffer Reaction.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer Reaction A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Because phosphoric acid has multiple. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Recipe can be automatically scaled by entering desired final volume. Phosphate acts best as a buffer in the reqions of ph near its three pk's: How do you prepare your. Phosphate Buffer Reaction.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer Reaction Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Recipe can be automatically scaled by entering desired final volume. For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction of the buffer components when a strong acid and a strong base is. Because phosphoric acid has multiple. Add 2.231 g of sodium. Phosphate Buffer Reaction.
From ar.inspiredpencil.com
Phosphate Buffer System Equation Phosphate Buffer Reaction Recipe can be automatically scaled by entering desired final volume. Add 2.231 g of sodium dihydrogen phosphate to the solution. Phosphate acts best as a buffer in the reqions of ph near its three pk's: Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. And pk 3 = 12.44. Monosodium phosphate, monohydrate and. Phosphate Buffer Reaction.
From www.bio-world.com
Sodium Phosphate Buffer 0.5M, pH 7.5 (7601549) bioWORLD Phosphate Buffer Reaction And pk 3 = 12.44. Pk 1 = 2.12, pk 2 = 7.21; Add 2.231 g of sodium dihydrogen phosphate to the solution. Because phosphoric acid has multiple. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. A phosphate buffer solution is a. Phosphate Buffer Reaction.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Phosphate Buffer Reaction Because phosphoric acid has multiple. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction of the buffer components when a strong acid and a strong base is. Add 2.231 g of sodium dihydrogen phosphate to the solution. How do you prepare your. Phosphate Buffer Reaction.
From bio-solution.co.kr
[BS021] 0.5M Sodium Phosphate Buffer, pH x.x Biosolution Phosphate Buffer Reaction Pk 1 = 2.12, pk 2 = 7.21; Add 2.231 g of sodium dihydrogen phosphate to the solution. Because phosphoric acid has multiple. And pk 3 = 12.44. Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. Add 11.555 g of sodium phosphate dibasic to the solution. Recipe can be automatically scaled by. Phosphate Buffer Reaction.
From www.researchgate.net
1 HNMR investigation of the reaction of ETZ with phosphate buffer (pH Phosphate Buffer Reaction Add 11.555 g of sodium phosphate dibasic to the solution. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Pk 1 = 2.12, pk 2 = 7.21; Recipe can be automatically scaled by entering desired final volume. Because phosphoric acid has multiple. Phosphate acts. Phosphate Buffer Reaction.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation Phosphate Buffer Reaction Recipe can be automatically scaled by entering desired final volume. Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Phosphate acts best as a buffer in the reqions of ph near its three pk's: And pk 3 =. Phosphate Buffer Reaction.
From www.chegg.com
For a buffer lab for a pH of 7.4 I need help with Phosphate Buffer Reaction Add 11.555 g of sodium phosphate dibasic to the solution. How do you prepare your phosphate buffer? Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. Add 2.231 g of sodium dihydrogen phosphate. Phosphate Buffer Reaction.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer Reaction How do you prepare your phosphate buffer? Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Add 2.231 g of sodium dihydrogen phosphate to the solution. Recipe can be automatically scaled by entering desired final volume. Because phosphoric acid has multiple. And pk 3 = 12.44. Add 11.555 g of sodium phosphate dibasic to the solution. Pk 1 =. Phosphate Buffer Reaction.
From www.youtube.com
Equation for K3PO4 + H2O (Potassium phosphate + Water) YouTube Phosphate Buffer Reaction Add 11.555 g of sodium phosphate dibasic to the solution. Recipe can be automatically scaled by entering desired final volume. How do you prepare your phosphate buffer? Add 2.231 g of sodium dihydrogen phosphate to the solution. Pk 1 = 2.12, pk 2 = 7.21; Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen. Phosphate Buffer Reaction.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download Phosphate Buffer Reaction For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction of the buffer components when a strong acid and a strong base is. Add 11.555 g of sodium phosphate dibasic to the solution. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Add 2.231 g of sodium dihydrogen phosphate to the solution.. Phosphate Buffer Reaction.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer Reaction Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Pk 1 = 2.12, pk 2 = 7.21; Recipe can be automatically scaled by entering desired final volume. Add 2.231 g of sodium dihydrogen phosphate to the solution. Add 11.555 g of sodium phosphate dibasic to the solution. A phosphate buffer solution is a handy buffer to have around, especially. Phosphate Buffer Reaction.
From www.slideserve.com
PPT AcidBase Equilibrium (Mono and Polyprotic) [Chapter 10,11 Phosphate Buffer Reaction For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction of the buffer components when a strong acid and a strong base is. Phosphate acts best as a buffer in the reqions of ph near its three pk's: Recipe can be automatically scaled by entering desired final volume. Phosphate buffer (ph 5.8 to. Phosphate Buffer Reaction.
From www.vrogue.co
Phosphate Buffer System Equation vrogue.co Phosphate Buffer Reaction Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Pk 1 = 2.12, pk 2 = 7.21; Add 2.231 g of sodium dihydrogen phosphate to the solution. How do you prepare your phosphate buffer? A phosphate buffer solution is a handy buffer to have around, especially for biological applications. And pk 3 = 12.44. Add 11.555 g of sodium. Phosphate Buffer Reaction.
From www.fishersci.com
MP Biomedicals Sodium Phosphate Buffer Sodium Phosphate Buffer Phosphate Buffer Reaction Recipe can be automatically scaled by entering desired final volume. For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction of the buffer components when a strong acid and a strong base is. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Gomori buffers, the most commonly. Phosphate Buffer Reaction.
From chemistry-europe.onlinelibrary.wiley.com
Influence of Phosphate Buffer on the Reaction of 3‐Nitrophenylboronic Phosphate Buffer Reaction Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. Add 11.555 g of sodium phosphate dibasic to the solution. Recipe can be automatically scaled by entering desired final volume. How do you prepare your phosphate buffer? For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction. Phosphate Buffer Reaction.
From www.slideserve.com
PPT Acid and Base Balance PowerPoint Presentation, free download ID Phosphate Buffer Reaction Add 2.231 g of sodium dihydrogen phosphate to the solution. Because phosphoric acid has multiple. And pk 3 = 12.44. Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Recipe can be automatically scaled by entering desired final. Phosphate Buffer Reaction.
From www.researchgate.net
Comparison of reactions in sodium phosphate buffer (pH 7.0) and Phosphate Buffer Reaction How do you prepare your phosphate buffer? For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction of the buffer components when a strong acid and a strong base is. And pk 3 = 12.44. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. A phosphate buffer solution is a handy buffer. Phosphate Buffer Reaction.
From www.bio-world.com
You are being redirected... Phosphate Buffer Reaction Because phosphoric acid has multiple. Add 2.231 g of sodium dihydrogen phosphate to the solution. Recipe can be automatically scaled by entering desired final volume. Pk 1 = 2.12, pk 2 = 7.21; And pk 3 = 12.44. For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction of the buffer components when. Phosphate Buffer Reaction.
From www.slideserve.com
PPT ACIDBASE BALANCE PowerPoint Presentation, free download ID6579634 Phosphate Buffer Reaction Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. Add 2.231 g of sodium dihydrogen phosphate to the solution. And pk 3 = 12.44. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction of the. Phosphate Buffer Reaction.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer Reaction Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Pk 1 = 2.12, pk 2 = 7.21; Recipe can be automatically scaled by entering desired final volume. Add 11.555 g of sodium phosphate dibasic to the solution. Gomori buffers, the most commonly used phosphate buffers, consist of a mixture. Phosphate Buffer Reaction.
From www.slideserve.com
PPT Fluid & Electrolyte Balance PowerPoint Presentation, free Phosphate Buffer Reaction How do you prepare your phosphate buffer? Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Add 2.231 g of sodium dihydrogen phosphate to the solution. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Recipe can be automatically scaled by entering desired final volume. Pk 1 = 2.12, pk 2 = 7.21; Because phosphoric acid has multiple.. Phosphate Buffer Reaction.
From operaresidences.com.au
Describe the role of the phosphate buffer system? Opera Residences Phosphate Buffer Reaction And pk 3 = 12.44. Recipe can be automatically scaled by entering desired final volume. Pk 1 = 2.12, pk 2 = 7.21; A phosphate buffer solution is a handy buffer to have around, especially for biological applications. How do you prepare your phosphate buffer? Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Phosphate buffer (ph 5.8 to. Phosphate Buffer Reaction.
From www.slideserve.com
PPT Chapter 24 Water, Electrolyte and AcidBase Balance PowerPoint Phosphate Buffer Reaction Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Add 2.231 g of sodium dihydrogen phosphate to the solution. Phosphate acts best as a buffer in the reqions of ph near its three pk's: How do you prepare your phosphate buffer? For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction of. Phosphate Buffer Reaction.
From dir.indiamart.com
Phosphate Buffered Saline PBS Solution Latest Price, Manufacturers Phosphate Buffer Reaction Add 11.555 g of sodium phosphate dibasic to the solution. Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. How do you prepare your phosphate buffer? Pk 1 = 2.12, pk 2 = 7.21; For each combination in exercise 4 that is a buffer, write the chemical equations for the reaction of the. Phosphate Buffer Reaction.
From cacheby.com
Himedia Phosphate Buffer, pH 8.0 캐시바이 Phosphate Buffer Reaction How do you prepare your phosphate buffer? Because phosphoric acid has multiple. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Phosphate acts best as a buffer in the reqions of ph near its three pk's: Recipe can be automatically scaled by entering desired final volume. Monosodium phosphate, monohydrate and its conjugate base disodium. Phosphate Buffer Reaction.
From www.researchgate.net
(a) Reaction between DFF and nbutylamine in potassium phosphate Phosphate Buffer Reaction And pk 3 = 12.44. Recipe can be automatically scaled by entering desired final volume. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Pk 1 = 2.12, pk 2 = 7.21; How do you prepare your phosphate buffer? Because phosphoric acid has multiple.. Phosphate Buffer Reaction.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer Reaction Add 2.231 g of sodium dihydrogen phosphate to the solution. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. How do you prepare your phosphate buffer? Recipe can be automatically scaled by entering desired final volume. For each combination in exercise 4 that is a buffer, write the chemical. Phosphate Buffer Reaction.
From philschatz.com
AcidBase Balance · Anatomy and Physiology Phosphate Buffer Reaction Add 11.555 g of sodium phosphate dibasic to the solution. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. For each combination in exercise 4 that is a. Phosphate Buffer Reaction.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer Reaction Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Because phosphoric acid has multiple. Gomori buffers, the most commonly used phosphate buffers, consist of a mixture of monobasic dihydrogen phosphate. Phosphate acts best as a buffer in the reqions of ph near its three pk's: A phosphate buffer solution is a handy buffer to have around, especially for biological. Phosphate Buffer Reaction.
From www.numerade.com
SOLVEDA biochemical reaction takes place in a 1.00 ml solution of 0. Phosphate Buffer Reaction Add 2.231 g of sodium dihydrogen phosphate to the solution. Phosphate acts best as a buffer in the reqions of ph near its three pk's: Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Pk 1 = 2.12, pk 2 = 7.21; How do. Phosphate Buffer Reaction.