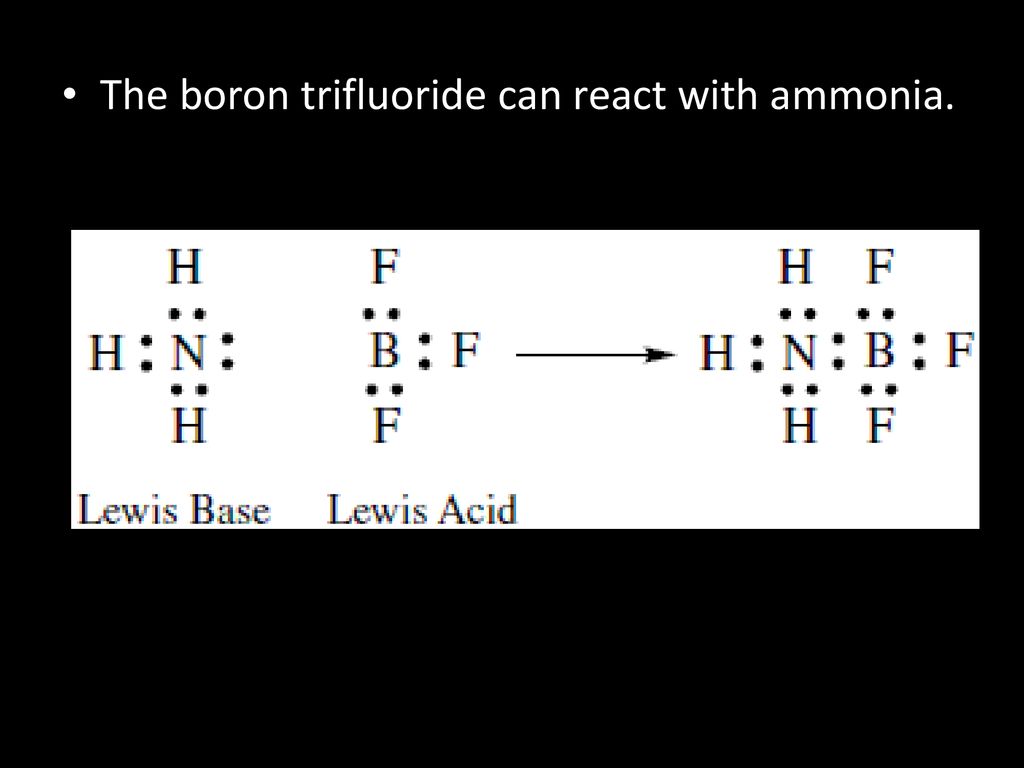
Boron Trifluoride Ammonia . a reaction between ammonia and boron trifluoride is given below: this question is asking us to identify the type of bond formed between ammonia and boron. N h 3 + b f 3 → h 3 n: As there is no hydrogen ion involved in this reaction,. The nitrogen atom has a lone pair and is an electron donor. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. (a) draw the lewis structure. ammonia (nh 3) and boron trifluoride (bf 3) are both chemical compounds that have unique properties and applications. boron trifluoride is the lewis acid, while ammonia is again the lewis base. Its molecular weight is 84.84 u. B f 3 identify the acid and base in. ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n.
from slideplayer.com
Its molecular weight is 84.84 u. As there is no hydrogen ion involved in this reaction,. a reaction between ammonia and boron trifluoride is given below: B f 3 identify the acid and base in. boron trifluoride is the lewis acid, while ammonia is again the lewis base. this question is asking us to identify the type of bond formed between ammonia and boron. (a) draw the lewis structure. ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n. N h 3 + b f 3 → h 3 n: ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7.
Lewis acids and bases. ppt download
Boron Trifluoride Ammonia ammonia (nh 3) and boron trifluoride (bf 3) are both chemical compounds that have unique properties and applications. (a) draw the lewis structure. this question is asking us to identify the type of bond formed between ammonia and boron. The nitrogen atom has a lone pair and is an electron donor. B f 3 identify the acid and base in. boron trifluoride is the lewis acid, while ammonia is again the lewis base. Its molecular weight is 84.84 u. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. As there is no hydrogen ion involved in this reaction,. N h 3 + b f 3 → h 3 n: a reaction between ammonia and boron trifluoride is given below: ammonia (nh 3) and boron trifluoride (bf 3) are both chemical compounds that have unique properties and applications. ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n.
From www.fishersci.com
Boron trifluoride etherate, ca. 48 BF3, AcroSeal, ACROS Organics Boron Trifluoride Ammonia this question is asking us to identify the type of bond formed between ammonia and boron. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. a reaction between ammonia and boron trifluoride is given below: The nitrogen atom has a lone pair and is an electron donor. ammonia boron. Boron Trifluoride Ammonia.
From slideplayer.com
Reactions involving Acids, Bases, & Salts ppt download Boron Trifluoride Ammonia this question is asking us to identify the type of bond formed between ammonia and boron. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. As there is no hydrogen ion involved in this reaction,. N h 3 + b f 3 → h 3 n: (a) draw the lewis structure.. Boron Trifluoride Ammonia.
From www.numerade.com
SOLVEDSuppose you carry out the following reaction of ammonia and Boron Trifluoride Ammonia B f 3 identify the acid and base in. boron trifluoride is the lewis acid, while ammonia is again the lewis base. this question is asking us to identify the type of bond formed between ammonia and boron. (a) draw the lewis structure. As there is no hydrogen ion involved in this reaction,. Its molecular weight is 84.84. Boron Trifluoride Ammonia.
From quizlet.com
Ammonia reacts with boron trifluoride to form a stable compo Quizlet Boron Trifluoride Ammonia (a) draw the lewis structure. Its molecular weight is 84.84 u. boron trifluoride is the lewis acid, while ammonia is again the lewis base. The nitrogen atom has a lone pair and is an electron donor. this question is asking us to identify the type of bond formed between ammonia and boron. a reaction between ammonia and. Boron Trifluoride Ammonia.
From www.researchgate.net
(PDF) Opening up new research lines in Lewis acid/base catalysis Boron Trifluoride Ammonia (a) draw the lewis structure. As there is no hydrogen ion involved in this reaction,. B f 3 identify the acid and base in. The nitrogen atom has a lone pair and is an electron donor. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. a reaction between ammonia and boron. Boron Trifluoride Ammonia.
From www.youtube.com
Dative (coordinate) covalent bonding between Boron Trifluoride and Boron Trifluoride Ammonia this question is asking us to identify the type of bond formed between ammonia and boron. ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n. a reaction between ammonia and boron trifluoride is given below: ammonia (nh 3) and boron trifluoride (bf 3) are both chemical compounds that have unique properties and applications.. Boron Trifluoride Ammonia.
From ar.inspiredpencil.com
Boron Tribromide Lewis Structure Boron Trifluoride Ammonia ammonia (nh 3) and boron trifluoride (bf 3) are both chemical compounds that have unique properties and applications. As there is no hydrogen ion involved in this reaction,. boron trifluoride is the lewis acid, while ammonia is again the lewis base. ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n. The nitrogen atom has. Boron Trifluoride Ammonia.
From solvedlib.com
Ammonia reacts with boron trifluoride to form a stabl… SolvedLib Boron Trifluoride Ammonia Its molecular weight is 84.84 u. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. The nitrogen atom has a lone pair and is an electron donor. ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n. As there is no hydrogen ion involved in this reaction,. this. Boron Trifluoride Ammonia.
From slideplayer.com
Lewis acids and bases. ppt download Boron Trifluoride Ammonia ammonia (nh 3) and boron trifluoride (bf 3) are both chemical compounds that have unique properties and applications. B f 3 identify the acid and base in. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. a reaction between ammonia and boron trifluoride is given below: boron trifluoride is. Boron Trifluoride Ammonia.
From www.pinterest.com
Molecular Orbital Diagram for Boron Trifluoride General Chemistry Boron Trifluoride Ammonia ammonia (nh 3) and boron trifluoride (bf 3) are both chemical compounds that have unique properties and applications. B f 3 identify the acid and base in. As there is no hydrogen ion involved in this reaction,. The nitrogen atom has a lone pair and is an electron donor. boron trifluoride is the lewis acid, while ammonia is. Boron Trifluoride Ammonia.
From www.mdpi.com
IJMS Free FullText Molecular Orbital and Density Functional Study Boron Trifluoride Ammonia ammonia (nh 3) and boron trifluoride (bf 3) are both chemical compounds that have unique properties and applications. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. B f 3 identify the acid and base in. a reaction between ammonia and boron trifluoride is given below: this question is. Boron Trifluoride Ammonia.
From www.tessshebaylo.com
Equation For Ammonia Reacting With Water Tessshebaylo Boron Trifluoride Ammonia B f 3 identify the acid and base in. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. a reaction between ammonia and boron trifluoride is given below: ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n. N h 3 + b f 3 → h 3. Boron Trifluoride Ammonia.
From slideplayer.com
Reactions involving Acids, Bases, & Salts ppt download Boron Trifluoride Ammonia N h 3 + b f 3 → h 3 n: boron trifluoride is the lewis acid, while ammonia is again the lewis base. this question is asking us to identify the type of bond formed between ammonia and boron. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. As. Boron Trifluoride Ammonia.
From www.numerade.com
VIDEO solution 24 3 5 6 9 10 Match with the correct name A. water B Boron Trifluoride Ammonia this question is asking us to identify the type of bond formed between ammonia and boron. B f 3 identify the acid and base in. As there is no hydrogen ion involved in this reaction,. Its molecular weight is 84.84 u. The nitrogen atom has a lone pair and is an electron donor. ammonia reacts with boron trifluoride. Boron Trifluoride Ammonia.
From www.nagwa.com
Question Video Understanding How Ammonia Molecules Bond Boron Boron Trifluoride Ammonia boron trifluoride is the lewis acid, while ammonia is again the lewis base. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. The nitrogen atom has a lone pair and is an electron donor. this question is asking us to identify the type of bond formed between ammonia and boron.. Boron Trifluoride Ammonia.
From www.chegg.com
Solved 6) When boron trifluoride reacts with ammonia, the Boron Trifluoride Ammonia As there is no hydrogen ion involved in this reaction,. B f 3 identify the acid and base in. The nitrogen atom has a lone pair and is an electron donor. a reaction between ammonia and boron trifluoride is given below: ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. N. Boron Trifluoride Ammonia.
From www.slideserve.com
PPT Ionic Bonding, NaCl PowerPoint Presentation, free download ID Boron Trifluoride Ammonia As there is no hydrogen ion involved in this reaction,. N h 3 + b f 3 → h 3 n: boron trifluoride is the lewis acid, while ammonia is again the lewis base. (a) draw the lewis structure. B f 3 identify the acid and base in. The nitrogen atom has a lone pair and is an electron. Boron Trifluoride Ammonia.
From thepresentation.ru
An introduction to bonding Boron Trifluoride Ammonia As there is no hydrogen ion involved in this reaction,. N h 3 + b f 3 → h 3 n: ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. Its molecular weight is 84.84 u. boron trifluoride is the lewis acid, while ammonia is again the lewis base. ammonia. Boron Trifluoride Ammonia.
From chempedia.info
Boron trifluoride reaction with ammonia Big Chemical Encyclopedia Boron Trifluoride Ammonia ammonia (nh 3) and boron trifluoride (bf 3) are both chemical compounds that have unique properties and applications. Its molecular weight is 84.84 u. boron trifluoride is the lewis acid, while ammonia is again the lewis base. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. (a) draw the lewis. Boron Trifluoride Ammonia.
From www.chegg.com
Solved Ammonia, NH3 and boron trifluoride, BFF3 are covalent Boron Trifluoride Ammonia a reaction between ammonia and boron trifluoride is given below: N h 3 + b f 3 → h 3 n: boron trifluoride is the lewis acid, while ammonia is again the lewis base. B f 3 identify the acid and base in. The nitrogen atom has a lone pair and is an electron donor. As there is. Boron Trifluoride Ammonia.
From www.numerade.com
Suppose you canry out the following reaction of ammonia and boron Boron Trifluoride Ammonia ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. The nitrogen atom has a lone pair and is an electron donor. Its molecular weight is 84.84 u. boron trifluoride is the lewis acid, while ammonia is again the lewis base. ammonia (nh 3) and boron trifluoride (bf 3) are both. Boron Trifluoride Ammonia.
From www.nagwa.com
Question Video Identifying the Lewis Acid in the Reaction of Ammonia Boron Trifluoride Ammonia N h 3 + b f 3 → h 3 n: As there is no hydrogen ion involved in this reaction,. this question is asking us to identify the type of bond formed between ammonia and boron. ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n. ammonia reacts with boron trifluoride to form a. Boron Trifluoride Ammonia.
From www.differencebetween.com
What is the Difference Between Ammonia and Boron Trifluoride Compare Boron Trifluoride Ammonia B f 3 identify the acid and base in. boron trifluoride is the lewis acid, while ammonia is again the lewis base. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. this question is asking us to identify the type of bond formed between ammonia and boron. The nitrogen atom. Boron Trifluoride Ammonia.
From www.youtube.com
How to Draw the Lewis Dot Structure for BrF3 Boron trifluoride YouTube Boron Trifluoride Ammonia The nitrogen atom has a lone pair and is an electron donor. a reaction between ammonia and boron trifluoride is given below: ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n. this question is asking us to identify the type of bond formed between ammonia and boron. Its molecular weight is 84.84 u. . Boron Trifluoride Ammonia.
From www.victoriana.com
Installieren Schöne Frau Herumlaufen boron trifluoride etherate Boron Trifluoride Ammonia ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. N h 3 + b f 3 → h 3 n: The nitrogen atom has a lone pair and is an electron donor. Its molecular weight is 84.84 u. . Boron Trifluoride Ammonia.
From www.chegg.com
Solved Boron trifluoride, BF3, can accept a pair of electron Boron Trifluoride Ammonia (a) draw the lewis structure. N h 3 + b f 3 → h 3 n: The nitrogen atom has a lone pair and is an electron donor. a reaction between ammonia and boron trifluoride is given below: this question is asking us to identify the type of bond formed between ammonia and boron. ammonia boron trifluoride. Boron Trifluoride Ammonia.
From www.youtube.com
Geometry of NH3 and BF3 Geometry of ammonia and Boron trifluoride Boron Trifluoride Ammonia B f 3 identify the acid and base in. ammonia (nh 3) and boron trifluoride (bf 3) are both chemical compounds that have unique properties and applications. a reaction between ammonia and boron trifluoride is given below: As there is no hydrogen ion involved in this reaction,. The nitrogen atom has a lone pair and is an electron. Boron Trifluoride Ammonia.
From www.chegg.com
Solved The reaction we are studying is the formation of an Boron Trifluoride Ammonia Its molecular weight is 84.84 u. (a) draw the lewis structure. boron trifluoride is the lewis acid, while ammonia is again the lewis base. a reaction between ammonia and boron trifluoride is given below: The nitrogen atom has a lone pair and is an electron donor. N h 3 + b f 3 → h 3 n: . Boron Trifluoride Ammonia.
From www.mdpi.com
IJMS Free FullText Molecular Orbital and Density Functional Study Boron Trifluoride Ammonia As there is no hydrogen ion involved in this reaction,. (a) draw the lewis structure. Its molecular weight is 84.84 u. a reaction between ammonia and boron trifluoride is given below: B f 3 identify the acid and base in. ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. The nitrogen. Boron Trifluoride Ammonia.
From chempedia.info
Boron trifluoride ammonia Big Chemical Encyclopedia Boron Trifluoride Ammonia this question is asking us to identify the type of bond formed between ammonia and boron. (a) draw the lewis structure. boron trifluoride is the lewis acid, while ammonia is again the lewis base. ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n. Its molecular weight is 84.84 u. a reaction between ammonia. Boron Trifluoride Ammonia.
From www.fishersci.com
Boron trifluoride, 99+, Thermo Scientific Chemicals, Quantity 100 g Boron Trifluoride Ammonia B f 3 identify the acid and base in. a reaction between ammonia and boron trifluoride is given below: ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n. (a) draw the lewis structure. boron trifluoride is the lewis acid, while ammonia is again the lewis base. this question is asking us to identify. Boron Trifluoride Ammonia.
From slideplayer.com
A guide for A level students KNOCKHARDY PUBLISHING ppt download Boron Trifluoride Ammonia ammonia reacts with boron trifluoride to form a stable compound, as we saw in section 8.7. As there is no hydrogen ion involved in this reaction,. (a) draw the lewis structure. The nitrogen atom has a lone pair and is an electron donor. this question is asking us to identify the type of bond formed between ammonia and. Boron Trifluoride Ammonia.
From www.nagwa.com
Question Video Classifying Ammonia and Boron Trifluoride during the Boron Trifluoride Ammonia B f 3 identify the acid and base in. a reaction between ammonia and boron trifluoride is given below: ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n. The nitrogen atom has a lone pair and is an electron donor. As there is no hydrogen ion involved in this reaction,. ammonia reacts with boron. Boron Trifluoride Ammonia.
From www.numerade.com
SOLVEDBoron trifluoride, BF3, reacts with ammonia, NH3, to form an Boron Trifluoride Ammonia boron trifluoride is the lewis acid, while ammonia is again the lewis base. Its molecular weight is 84.84 u. N h 3 + b f 3 → h 3 n: a reaction between ammonia and boron trifluoride is given below: As there is no hydrogen ion involved in this reaction,. ammonia reacts with boron trifluoride to form. Boron Trifluoride Ammonia.
From www.numerade.com
SOLVEDWhen boron trifluoride reacts with ammonia, the following Boron Trifluoride Ammonia Its molecular weight is 84.84 u. (a) draw the lewis structure. B f 3 identify the acid and base in. ammonia boron trifluoride or trifluoroborane ammonia has a molecular formula of bf3h3n. a reaction between ammonia and boron trifluoride is given below: ammonia (nh 3) and boron trifluoride (bf 3) are both chemical compounds that have unique. Boron Trifluoride Ammonia.