Icd Extraction Guidelines . Recommendations for removal of infected cied 1. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. Heart rhythm society expert consensus on facilities, training, indications, and patient. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. the fda strongly recommended against the routine extraction of these leads and stated in the recall notice that “neither fda,.
from www.semanticscholar.org
Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. the fda strongly recommended against the routine extraction of these leads and stated in the recall notice that “neither fda,. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. Recommendations for removal of infected cied 1. Heart rhythm society expert consensus on facilities, training, indications, and patient. Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal.
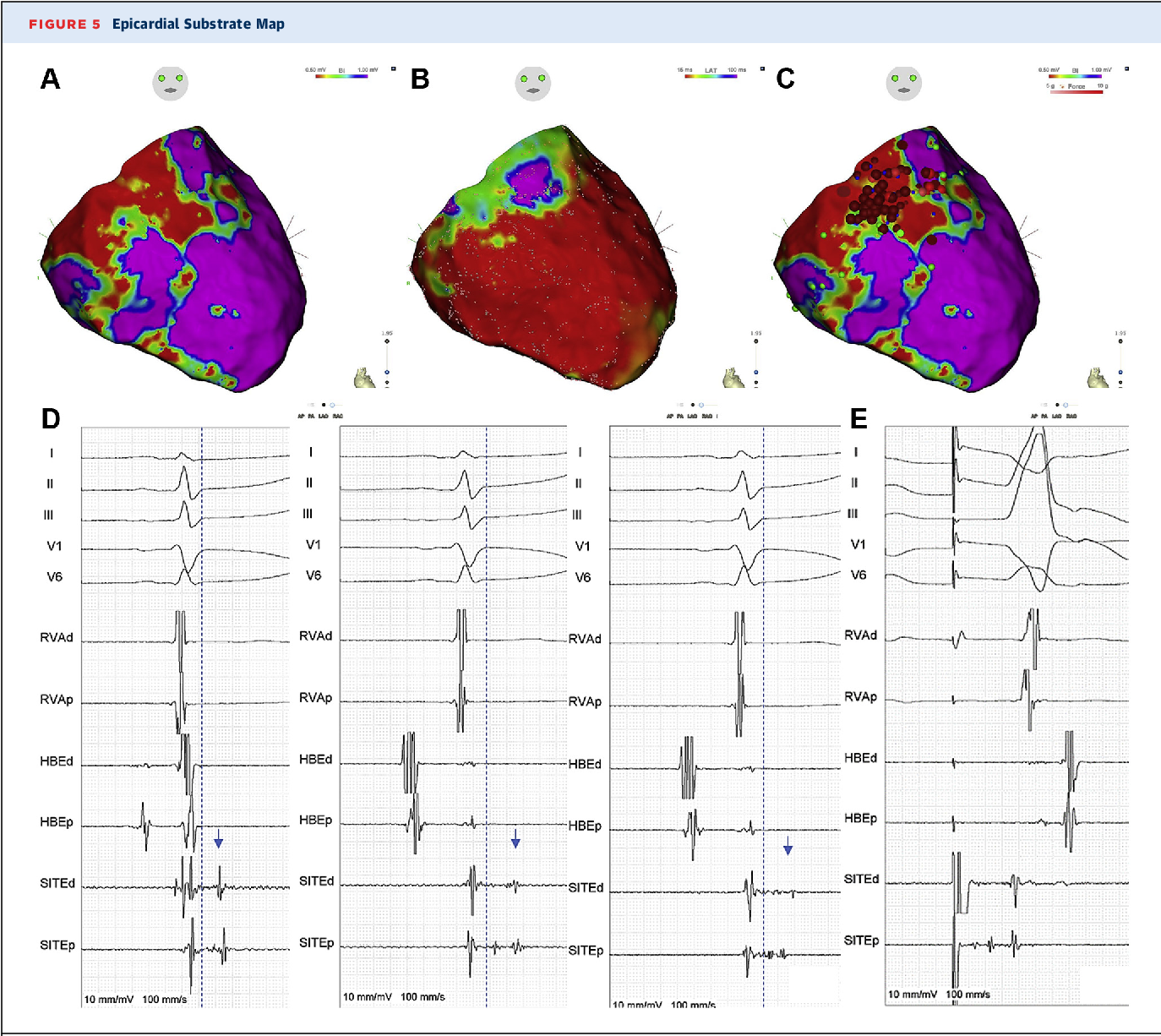
Figure 5 from Subcutaneous ICD Implantation and Catheter Ablation
Icd Extraction Guidelines the fda strongly recommended against the routine extraction of these leads and stated in the recall notice that “neither fda,. Recommendations for removal of infected cied 1. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. the fda strongly recommended against the routine extraction of these leads and stated in the recall notice that “neither fda,. Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. Heart rhythm society expert consensus on facilities, training, indications, and patient.
From www.researchgate.net
A flow chart of data extraction process. ICD10AM International Icd Extraction Guidelines Recommendations for removal of infected cied 1. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. Complete device and lead removal. Icd Extraction Guidelines.
From childrensnational.org
Pediatric Pacing and ICD Lead Extraction Icd Extraction Guidelines the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. Recommendations for removal of infected cied 1. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. the fda. Icd Extraction Guidelines.
From www.researchgate.net
(PDF) Systematic Review on SICD Lead Extraction Icd Extraction Guidelines Heart rhythm society expert consensus on facilities, training, indications, and patient. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. Complete. Icd Extraction Guidelines.
From icdlist.com
ICD10PCS Procedure Code 10D00Z1 Extraction of Products of Icd Extraction Guidelines Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. Heart rhythm society expert consensus on facilities, training, indications, and patient. the 2015 hrs/ehra/aphrs/solaece expert. Icd Extraction Guidelines.
From www.slideshare.net
Icd lead extraction ba nov 2012 final Icd Extraction Guidelines Recommendations for removal of infected cied 1. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. Complete device and lead removal is recommended for all. Icd Extraction Guidelines.
From www.semanticscholar.org
Figure 5 from Subcutaneous ICD Implantation and Catheter Ablation Icd Extraction Guidelines the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. Recommendations for removal of infected cied 1. Heart rhythm society expert consensus on facilities, training, indications, and patient. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. the 2015. Icd Extraction Guidelines.
From www.ahajournals.org
ACC/AHA Guidelines for the Management of Patients With STElevation Icd Extraction Guidelines the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. Recommendations for removal of infected cied 1. Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. the fda strongly recommended against the routine. Icd Extraction Guidelines.
From www.researchgate.net
Diagram of sampling and data extraction process. ICD10 the 10th Icd Extraction Guidelines Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. Recommendations for removal of infected cied 1. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. Heart rhythm society. Icd Extraction Guidelines.
From www.mdpi.com
JCM Free FullText Systematic Review on SICD Lead Extraction Icd Extraction Guidelines Recommendations for removal of infected cied 1. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. the statement focuses on identifying the presence of lead malfunction, deciding on whether. Icd Extraction Guidelines.
From www.heartrhythmjournal.com
BPO02206 FEMORAL ICD EXTRACTION Heart Rhythm Icd Extraction Guidelines the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. Heart rhythm society expert consensus on facilities, training, indications, and patient. Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. Recommendations for removal of infected cied 1. Complete device and lead removal is. Icd Extraction Guidelines.
From health.ucsd.edu
Lead Extraction UC San Diego Health Icd Extraction Guidelines Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. Recommendations for removal of infected cied 1. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. Heart rhythm society. Icd Extraction Guidelines.
From www.researchgate.net
Study flowchart. AMI indicates acute myocardial infarction; ET Icd Extraction Guidelines Heart rhythm society expert consensus on facilities, training, indications, and patient. Recommendations for removal of infected cied 1. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. the fda strongly recommended against the routine extraction of these leads and stated in the recall notice that. Icd Extraction Guidelines.
From childrensnational.org
Pediatric Pacing and ICD Lead Extraction Icd Extraction Guidelines the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. the fda strongly recommended against the routine extraction of these leads and stated in the recall notice that “neither fda,. Heart rhythm society expert consensus on facilities, training, indications, and patient. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or. Icd Extraction Guidelines.
From www.ahajournals.org
HRS/ACC/AHA Expert Consensus Statement on the Use of Implantable Icd Extraction Guidelines Recommendations for removal of infected cied 1. the fda strongly recommended against the routine extraction of these leads and stated in the recall notice that “neither fda,. Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. the statement focuses on identifying the presence of lead malfunction, deciding on whether. Icd Extraction Guidelines.
From www.researchgate.net
(PDF) Procedure, management, and of subcutaneous implantable Icd Extraction Guidelines Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract. Icd Extraction Guidelines.
From www.researchgate.net
(PDF) A hybrid ICD extraction approach using laser and transfemoral Icd Extraction Guidelines Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. Heart rhythm society expert consensus on facilities, training, indications, and patient. Complete device and lead removal. Icd Extraction Guidelines.
From www.researchgate.net
(PDF) A Questionable Indication For ICD Extraction After Successful VT Icd Extraction Guidelines Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. Recommendations for removal of infected cied 1. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. Heart rhythm society expert consensus on facilities, training, indications, and patient. the statement focuses on identifying the presence of lead. Icd Extraction Guidelines.
From www.researchgate.net
Current ICD and CRT indications according to the ESC guidelines Icd Extraction Guidelines Recommendations for removal of infected cied 1. Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. Heart rhythm society expert consensus on facilities, training, indications, and patient. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. Complete device and. Icd Extraction Guidelines.
From www.semanticscholar.org
Figure 2 from A hybrid ICD extraction approach using laser and Icd Extraction Guidelines Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. the fda strongly recommended against the routine extraction of these leads and stated in the recall notice that “neither fda,. Recommendations for removal of infected cied 1. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. Heart rhythm society expert consensus. Icd Extraction Guidelines.
From www.mdedge.com
Extravascular ICD surpasses goals in pivotal trial MDedge Cardiology Icd Extraction Guidelines Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. the fda strongly recommended against the routine extraction of. Icd Extraction Guidelines.
From www.researchgate.net
(PDF) Endovascular extraction techniques for pacemaker and ICD lead Icd Extraction Guidelines the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. Recommendations for removal of infected cied 1. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. Complete device and. Icd Extraction Guidelines.
From giofjqtmn.blob.core.windows.net
Icd10Pcs Code For Cataract Extraction With Iol Implant at Brian Babb blog Icd Extraction Guidelines the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. the fda strongly recommended against the routine extraction of these leads. Icd Extraction Guidelines.
From www.cmajopen.ca
Validity and utility of ICD10 administrative health data for Icd Extraction Guidelines Heart rhythm society expert consensus on facilities, training, indications, and patient. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or. Icd Extraction Guidelines.
From statcardiologist.com
Indications for ICD Stat Cardiologist Icd Extraction Guidelines the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. the fda strongly recommended against the routine extraction of these leads and stated in the recall notice that “neither fda,.. Icd Extraction Guidelines.
From www.youtube.com
SICD Implantation 3Incision Technique with EMBLEM 3501 Electrode Icd Extraction Guidelines the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. Recommendations for removal of infected cied 1. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. Complete device and lead removal is recommended for all. Icd Extraction Guidelines.
From uktraumaprotocol.blogspot.com
UK Trauma Protocol Manual Bacteremia (Antimicrobial Duration and Icd Extraction Guidelines Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. . Icd Extraction Guidelines.
From capturebilling.com
Quick Guide to ICD10CM 2018 Myocardial Infarction Guideline Changes Icd Extraction Guidelines Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. the fda strongly recommended against the routine extraction of these leads and stated in the recall notice that “neither fda,. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. Heart rhythm society. Icd Extraction Guidelines.
From medshun.com
Demystifying The Icd10 Code For Wisdom Tooth Extraction A Complete Icd Extraction Guidelines the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. the 2015 hrs/ehra/aphrs/solaece expert consensus statement on optimal. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. Heart rhythm society expert consensus on facilities, training, indications, and. Icd Extraction Guidelines.
From www.researchgate.net
Indications of ICD implantation in the 2011 ACCF/AHA guideline (left Icd Extraction Guidelines the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. the fda strongly recommended against the routine extraction of these leads and stated in the recall notice that “neither fda,. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular. Icd Extraction Guidelines.
From www.jacc.org
Ventricular Arrhythmia and Sudden Cardiac Death Prevention Guideline Icd Extraction Guidelines Heart rhythm society expert consensus on facilities, training, indications, and patient. Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. Complete device and lead removal is recommended for all patients with definite cied. Icd Extraction Guidelines.
From www.researchgate.net
Application in practice of the algorithm of management and Icd Extraction Guidelines Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. Heart rhythm society expert consensus on facilities, training, indications, and patient. the fda strongly recommended against the routine extraction of these leads and stated in the recall notice that “neither fda,. the statement focuses on identifying the presence of lead. Icd Extraction Guidelines.
From www.slideserve.com
PPT ICD10TM Classification Health Intervention PowerPoint Icd Extraction Guidelines the fda strongly recommended against the routine extraction of these leads and stated in the recall notice that “neither fda,. Recommendations for removal of infected cied 1. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. the statement focuses on identifying the presence of lead malfunction, deciding on whether. Icd Extraction Guidelines.
From childrensnational.org
Pediatric Pacing and ICD Lead Extraction Icd Extraction Guidelines Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to. Recommendations for removal of infected cied 1. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or. Icd Extraction Guidelines.
From www.heartrhythmjournal.com
BPO05052 INDICATIONS FOR SUBCUTANEOUS ICD EXTRACTIONS A SINGLE Icd Extraction Guidelines the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. Complete device and lead removal is recommended for all patients with definite cied infection, as evidenced by valvular and/or lead endocarditis or sepsis. Heart rhythm society expert consensus on facilities, training, indications, and patient. the 2015 hrs/ehra/aphrs/solaece expert. Icd Extraction Guidelines.
From www.researchgate.net
Data extraction algorithm. ICD9CM, International Classification of Icd Extraction Guidelines Recommendations for removal of infected cied 1. Complete device and lead removal is recommended for all patients with ciem pocket infection, as evidenced by abscess. the statement focuses on identifying the presence of lead malfunction, deciding on whether to abandon or to extract a lead. Heart rhythm society expert consensus on facilities, training, indications, and patient. Complete device and. Icd Extraction Guidelines.