Sources Of Error In Calorimetry Experiment . learn why all science experiments have error, how to calculate it, and the sources and types of errors you. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. To apply these δh δ h. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to the. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry.
from studylib.net
Aside from possible sources of error, another limitation involves the kinds of reactions you can study. explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to the. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. learn why all science experiments have error, how to calculate it, and the sources and types of errors you. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. To apply these δh δ h.
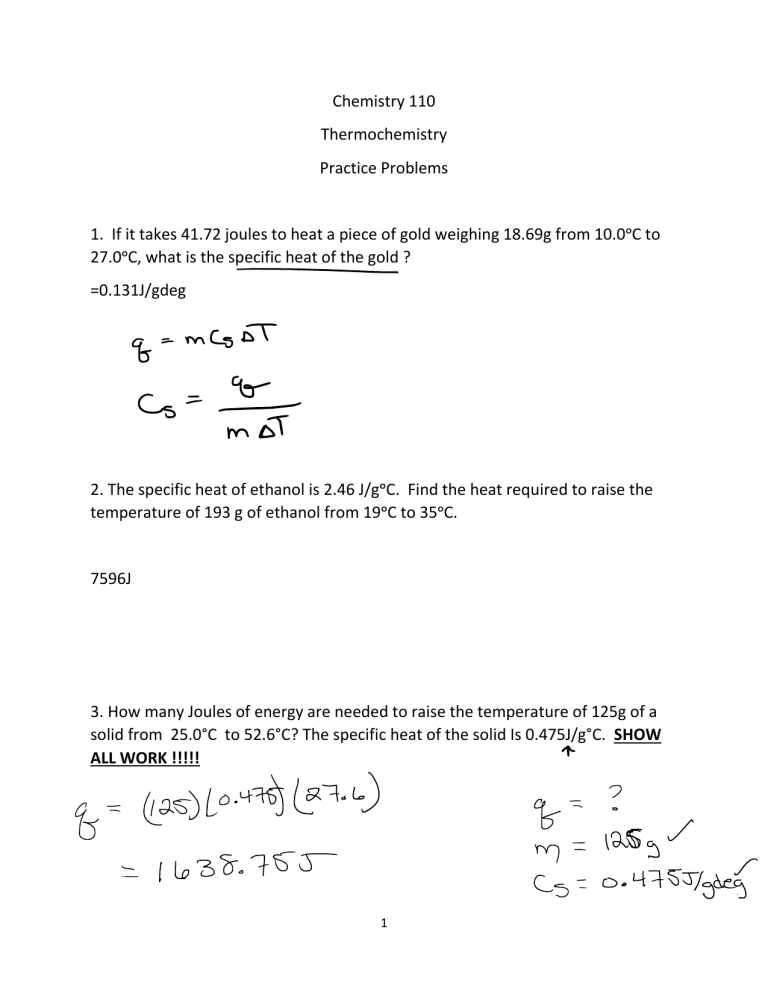
Calorimetry Experiment and Practice Problems for Virtual Lab Key
Sources Of Error In Calorimetry Experiment To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. learn why all science experiments have error, how to calculate it, and the sources and types of errors you. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. To apply these δh δ h. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to the. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Sources Of Error In Calorimetry Experiment Aside from possible sources of error, another limitation involves the kinds of reactions you can study. explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to the. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. learn. Sources Of Error In Calorimetry Experiment.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Sources Of Error In Calorimetry Experiment learn why all science experiments have error, how to calculate it, and the sources and types of errors you. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. revision notes on. Sources Of Error In Calorimetry Experiment.
From www.slideshare.net
4 Calorimetry Sources Of Error In Calorimetry Experiment explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to the. To apply these δh δ h. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by. Sources Of Error In Calorimetry Experiment.
From www.numerade.com
Name three possible sources of error in a calorimetry experiment Sources Of Error In Calorimetry Experiment To apply these δh δ h. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to the. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate. Sources Of Error In Calorimetry Experiment.
From www.scribd.com
EXPERIMENT 302 Heat and Calorimetry Analysis Sources of Error PDF Sources Of Error In Calorimetry Experiment to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. learn why all science experiments have error, how to calculate it, and the sources and types of errors you. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. . Sources Of Error In Calorimetry Experiment.
From studyfinder.org
Unveiling the Secrets of Experiment 25 Calorimetry Prelab Answers Sources Of Error In Calorimetry Experiment learn why all science experiments have error, how to calculate it, and the sources and types of errors you. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to. Sources Of Error In Calorimetry Experiment.
From www.slideserve.com
PPT Heat of Combustion PowerPoint Presentation, free download ID Sources Of Error In Calorimetry Experiment to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. To apply these δh δ h. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. explain clearly how the two errors below would increase or decrease your calculated value. Sources Of Error In Calorimetry Experiment.
From www.numerade.com
SOLVED Name three possible sources of error in a calorimetry experiment. Sources Of Error In Calorimetry Experiment revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. to experimentally measure. Sources Of Error In Calorimetry Experiment.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Sources Of Error In Calorimetry Experiment To apply these δh δ h. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based. Sources Of Error In Calorimetry Experiment.
From www.scribd.com
Experiment 1 Bomb Calorimetry Calorimetry Heat Sources Of Error In Calorimetry Experiment Aside from possible sources of error, another limitation involves the kinds of reactions you can study. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. learn why all science. Sources Of Error In Calorimetry Experiment.
From www.slideshare.net
4 Calorimetry Sources Of Error In Calorimetry Experiment explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to the. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. To apply these δh δ h. To observe and measure heat exchanged in a chemical reaction and to come. Sources Of Error In Calorimetry Experiment.
From exyqlzddm.blob.core.windows.net
Calorimeter Examples Chemistry at Sima Montgomery blog Sources Of Error In Calorimetry Experiment To apply these δh δ h. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to the. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by. Sources Of Error In Calorimetry Experiment.
From studylib.net
PPT Calorimetry Sources Of Error In Calorimetry Experiment explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to the. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. To apply. Sources Of Error In Calorimetry Experiment.
From www.researchgate.net
Temperature measurements during a typical calorimetry experiment Sources Of Error In Calorimetry Experiment To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. To apply these δh δ h. learn why all science experiments have error, how to calculate it, and the sources and types of errors you. Aside from possible sources of error, another limitation involves the kinds of reactions you. Sources Of Error In Calorimetry Experiment.
From www.scribd.com
Calorimetry Experiment Lab Report PDF Sodium Hydroxide Sources Of Error In Calorimetry Experiment To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. . Sources Of Error In Calorimetry Experiment.
From studylib.net
5.1 b) CALORIMETRY Sources Of Error In Calorimetry Experiment To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. To apply these δh δ h. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. Aside from possible sources of error, another limitation involves the kinds of reactions you. Sources Of Error In Calorimetry Experiment.
From hxekemegs.blob.core.windows.net
Calorimetry Chemistry Experiment at Robert Montague blog Sources Of Error In Calorimetry Experiment To apply these δh δ h. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. explain clearly how the two errors below would increase or decrease your calculated value for specific heat. Sources Of Error In Calorimetry Experiment.
From www.researchgate.net
Sources of error in calorimetry measurements Download Scientific Diagram Sources Of Error In Calorimetry Experiment Aside from possible sources of error, another limitation involves the kinds of reactions you can study. learn why all science experiments have error, how to calculate it, and the sources and types of errors you. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. explain clearly how. Sources Of Error In Calorimetry Experiment.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Sources Of Error In Calorimetry Experiment learn why all science experiments have error, how to calculate it, and the sources and types of errors you. To apply these δh δ h. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based. Sources Of Error In Calorimetry Experiment.
From kaffee.50webs.com
Lab Calorimetry Sources Of Error In Calorimetry Experiment Aside from possible sources of error, another limitation involves the kinds of reactions you can study. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. learn why all science experiments have error, how to calculate it, and the sources and types of errors you. revision notes on. Sources Of Error In Calorimetry Experiment.
From www.youtube.com
R1.1.4 What are the sources of error in calorimetry? YouTube Sources Of Error In Calorimetry Experiment Aside from possible sources of error, another limitation involves the kinds of reactions you can study. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. To apply these δh δ h. learn why all science experiments have error, how to calculate it, and the sources and types of. Sources Of Error In Calorimetry Experiment.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Sources Of Error In Calorimetry Experiment to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to the. revision notes. Sources Of Error In Calorimetry Experiment.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Sources Of Error In Calorimetry Experiment revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. To apply these δh. Sources Of Error In Calorimetry Experiment.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Sources Of Error In Calorimetry Experiment revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. To apply these δh δ h. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. Aside from possible sources of error, another limitation involves the kinds of reactions you. Sources Of Error In Calorimetry Experiment.
From exytcmfmb.blob.core.windows.net
Calorimetry Energy Content Of Food at Tim Rife blog Sources Of Error In Calorimetry Experiment learn why all science experiments have error, how to calculate it, and the sources and types of errors you. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. Aside. Sources Of Error In Calorimetry Experiment.
From www.nagwa.com
Question Video Identifying the Factor That Does Not Need to Be Held Sources Of Error In Calorimetry Experiment revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. learn why all science experiments have error, how to calculate it, and the sources and types of errors you. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous.. Sources Of Error In Calorimetry Experiment.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Sources Of Error In Calorimetry Experiment to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. To apply these δh δ h. learn why all science experiments have error, how to calculate it, and the sources. Sources Of Error In Calorimetry Experiment.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Sources Of Error In Calorimetry Experiment revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. To apply these δh δ h. Aside from possible sources of error, another limitation involves the kinds of reactions you can. Sources Of Error In Calorimetry Experiment.
From courses.lumenlearning.com
Calorimetry Chemistry Sources Of Error In Calorimetry Experiment To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring. Sources Of Error In Calorimetry Experiment.
From fyoaynlld.blob.core.windows.net
Principle Of Calorimetry In Biochemistry at Doris Hogan blog Sources Of Error In Calorimetry Experiment learn why all science experiments have error, how to calculate it, and the sources and types of errors you. explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to the. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save. Sources Of Error In Calorimetry Experiment.
From www.studocu.com
Calorimetry Experiment 25 Calorimetry To determine the specific heat Sources Of Error In Calorimetry Experiment To apply these δh δ h. learn why all science experiments have error, how to calculate it, and the sources and types of errors you. explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to the. Aside from possible sources of error, another limitation involves the kinds of. Sources Of Error In Calorimetry Experiment.
From www.slideserve.com
PPT Chapter 14 Heat PowerPoint Presentation, free download ID6832402 Sources Of Error In Calorimetry Experiment To apply these δh δ h. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure. Sources Of Error In Calorimetry Experiment.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Sources Of Error In Calorimetry Experiment To apply these δh δ h. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. Aside from possible sources of error, another limitation involves the kinds of reactions you can study. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my. Sources Of Error In Calorimetry Experiment.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Sources Of Error In Calorimetry Experiment to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring to. Sources Of Error In Calorimetry Experiment.
From studylib.net
Calorimetry Experiment and Practice Problems for Virtual Lab Key Sources Of Error In Calorimetry Experiment To observe and measure heat exchanged in a chemical reaction and to come up with appropriate inferences based on previous. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. explain clearly how the two errors below would increase or decrease your calculated value for specific heat by referring. Sources Of Error In Calorimetry Experiment.