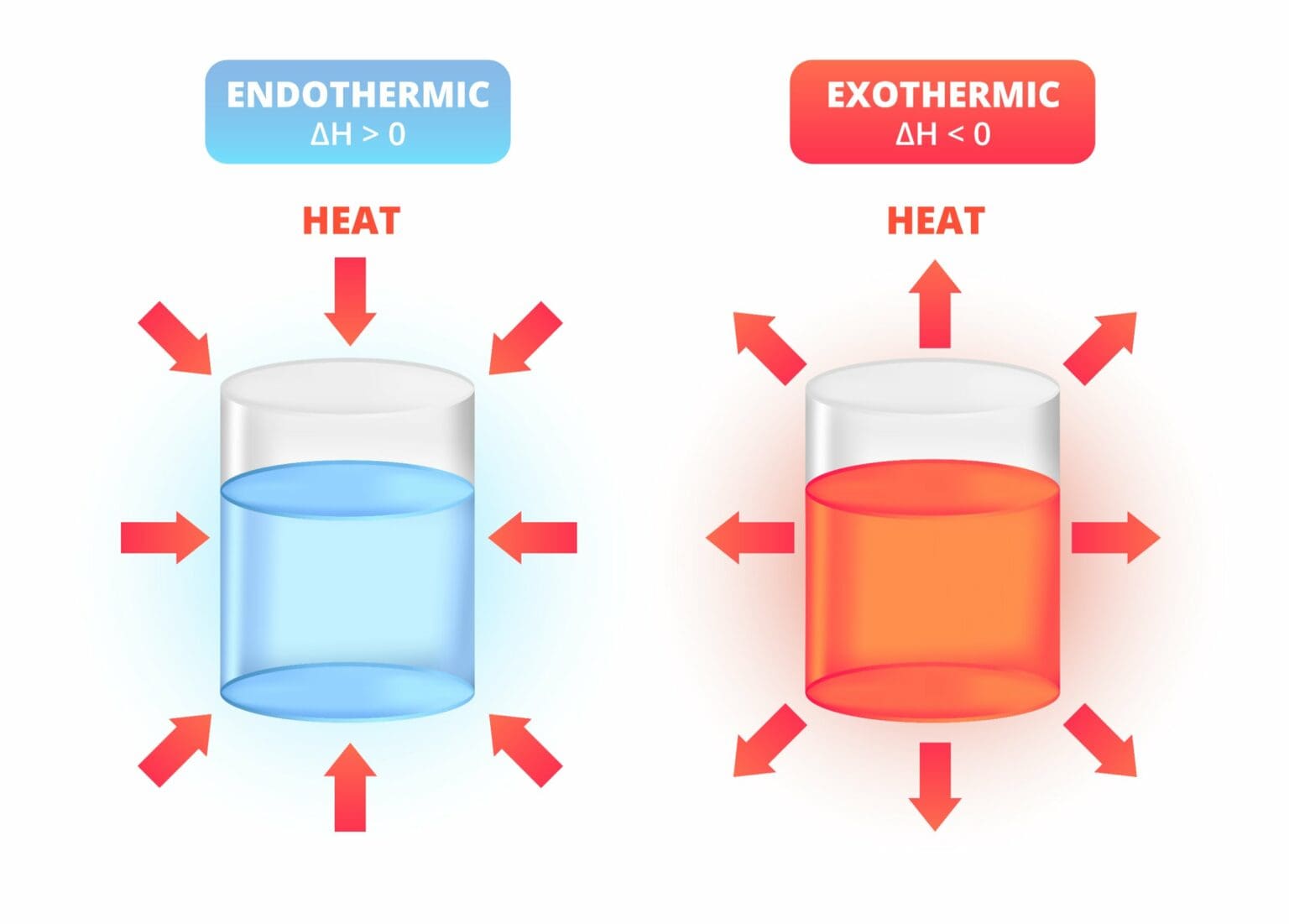
Is Shaking A Cold Pack Endothermic Or Exothermic . In an endothermic reaction, a substance takes heat. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. An exothermic chemical reaction causes an increase in temperature (of the surroundings). The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. As for the cold pak, that is an endothermic reaction. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions.
from online-learning-college.com
In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. An exothermic chemical reaction causes an increase in temperature (of the surroundings). In an endothermic reaction, a substance takes heat. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. As for the cold pak, that is an endothermic reaction.
Exothermic and endothermic reactions Key differences
Is Shaking A Cold Pack Endothermic Or Exothermic Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. In an endothermic reaction, a substance takes heat. An exothermic chemical reaction causes an increase in temperature (of the surroundings). As for the cold pak, that is an endothermic reaction. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding.
From slideplayer.com
CHAPTER 15 and 16 SOLUTIONS. ppt download Is Shaking A Cold Pack Endothermic Or Exothermic As for the cold pak, that is an endothermic reaction. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. In an endothermic reaction, a substance takes heat. In the. Is Shaking A Cold Pack Endothermic Or Exothermic.
From slideplayer.com
Endothermic and Exothermic Reactions ppt download Is Shaking A Cold Pack Endothermic Or Exothermic The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases.. Is Shaking A Cold Pack Endothermic Or Exothermic.
From question.pandai.org
Endothermic and exothermic reactions Is Shaking A Cold Pack Endothermic Or Exothermic In an endothermic reaction, a substance takes heat. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. In the course of an endothermic. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.youtube.com
Hot/Cold Pack Endothermic and Exothermic Reactions (4/11) YouTube Is Shaking A Cold Pack Endothermic Or Exothermic In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Chapter 7 Hot & Cold Packs PowerPoint Presentation, free Is Shaking A Cold Pack Endothermic Or Exothermic The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. As for the cold pak, that is an endothermic reaction. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. Learn about the chemical reactions used to make. Is Shaking A Cold Pack Endothermic Or Exothermic.
From taylorecchung.blogspot.com
Cold Pack Endothermic or Exothermic TaylorecChung Is Shaking A Cold Pack Endothermic Or Exothermic As for the cold pak, that is an endothermic reaction. An exothermic chemical reaction causes an increase in temperature (of the surroundings). In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. The changes in energy that occur during a chemical reaction can be seen by examining the. Is Shaking A Cold Pack Endothermic Or Exothermic.
From teachingscience.us
Exothermic and Endothermic Reactions Is Shaking A Cold Pack Endothermic Or Exothermic The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. As for the cold pak, that is an endothermic reaction. These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. Learn about the chemical. Is Shaking A Cold Pack Endothermic Or Exothermic.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Is Shaking A Cold Pack Endothermic Or Exothermic In an endothermic reaction, a substance takes heat. An exothermic chemical reaction causes an increase in temperature (of the surroundings). As for the cold pak, that is an endothermic reaction. These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. Learn about the chemical. Is Shaking A Cold Pack Endothermic Or Exothermic.
From junioratobrien.blogspot.com
JunioratObrien Is Shaking A Cold Pack Endothermic Or Exothermic The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. As for the cold pak, that is an endothermic. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.sciencebuddies.org
Cold Pack Chemistry Exploring Endothermic and Exothermic Reactions Is Shaking A Cold Pack Endothermic Or Exothermic These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. In. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Chapter 7 Hot & Cold Packs PowerPoint Presentation ID2507233 Is Shaking A Cold Pack Endothermic Or Exothermic An exothermic chemical reaction causes an increase in temperature (of the surroundings). Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. In an endothermic reaction, a substance takes heat. The changes in energy. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Is Shaking A Cold Pack Endothermic Or Exothermic Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. In an endothermic reaction, a substance takes heat. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. In the course of an endothermic process,. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Is Shaking A Cold Pack Endothermic Or Exothermic Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. An exothermic chemical reaction causes an increase in temperature (of the surroundings). The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. As for the cold pak, that is an endothermic reaction. In an endothermic reaction,. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Hot Packs and Cold Packs PowerPoint Presentation, free download Is Shaking A Cold Pack Endothermic Or Exothermic Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. An exothermic chemical reaction causes an increase in temperature (of the surroundings). As for the cold pak, that is an endothermic reaction. These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or. Is Shaking A Cold Pack Endothermic Or Exothermic.
From brooklynewtortega.blogspot.com
Cold Pack Endothermic or Exothermic BrooklynewtOrtega Is Shaking A Cold Pack Endothermic Or Exothermic In an endothermic reaction, a substance takes heat. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. As for the cold pak, that is an endothermic reaction. These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. The changes. Is Shaking A Cold Pack Endothermic Or Exothermic.
From courses.lumenlearning.com
The Dissolving Process CHEM 1305 Introductory Chemistry Is Shaking A Cold Pack Endothermic Or Exothermic Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. The changes in energy that occur during a chemical reaction can be seen by examining. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.thinkswap.com
Endothermic Reaction Practical (Science of Cold Packs) Chemistry Is Shaking A Cold Pack Endothermic Or Exothermic Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. An exothermic chemical reaction causes an increase in temperature (of the surroundings). In the course of an endothermic process, the system gains heat from the surroundings and. Is Shaking A Cold Pack Endothermic Or Exothermic.
From slideplayer.com
Chemical Change Chapter 3 Section ppt download Is Shaking A Cold Pack Endothermic Or Exothermic In an endothermic reaction, a substance takes heat. Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Exothermic. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.thoughtco.com
Make a Cold Pack from Hot Ice Is Shaking A Cold Pack Endothermic Or Exothermic The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. An exothermic chemical reaction causes an increase in temperature (of the surroundings). Exothermic reactions transfer energy to the. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.gauthmath.com
Label the following image of a cold pack to show the movement of Is Shaking A Cold Pack Endothermic Or Exothermic The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. In an endothermic reaction, a substance takes heat. An exothermic chemical reaction causes an increase in temperature (of the surroundings). In the course of an endothermic. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Is Shaking A Cold Pack Endothermic Or Exothermic In an endothermic reaction, a substance takes heat. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Exothermic reactions transfer energy to the surroundings and the temperature. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Hot Packs and Cold Packs PowerPoint Presentation, free download Is Shaking A Cold Pack Endothermic Or Exothermic In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. As for the cold pak, that is an endothermic reaction. Learn about the chemical reactions used to make cold packs and which chemicals to. Is Shaking A Cold Pack Endothermic Or Exothermic.
From slideplayer.com
Energy in Reactions L.O To know what happens in an endothermic and Is Shaking A Cold Pack Endothermic Or Exothermic An exothermic chemical reaction causes an increase in temperature (of the surroundings). These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. As for the cold pak, that is an endothermic reaction. Learn about the chemical reactions used to make cold packs and which. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.numerade.com
SOLVED 4. a) How does a cold pack work? Does it use an endothermic or Is Shaking A Cold Pack Endothermic Or Exothermic The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. As for the cold pak, that is an endothermic reaction. Learn about the chemical reactions used to make cold packs and which chemicals to mix to. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT CHEMISTRY 122 PowerPoint Presentation, free download ID6314666 Is Shaking A Cold Pack Endothermic Or Exothermic These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. In the course of an endothermic process, the system gains heat from the surroundings. Is Shaking A Cold Pack Endothermic Or Exothermic.
From mylaldnorman.blogspot.com
Cold Pack Endothermic or Exothermic MylaldNorman Is Shaking A Cold Pack Endothermic Or Exothermic The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases.. Is Shaking A Cold Pack Endothermic Or Exothermic.
From www.slideserve.com
PPT Enthalpy PowerPoint Presentation, free download ID6682933 Is Shaking A Cold Pack Endothermic Or Exothermic In an endothermic reaction, a substance takes heat. As for the cold pak, that is an endothermic reaction. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. Learn about. Is Shaking A Cold Pack Endothermic Or Exothermic.
From slidetodoc.com
Thermodynamics Unit 10 Endothermic vs Exothermic Endo chemical Is Shaking A Cold Pack Endothermic Or Exothermic Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. An exothermic chemical reaction causes an increase in temperature (of the surroundings). In an endothermic reaction, a substance takes heat. These cold packs have. Is Shaking A Cold Pack Endothermic Or Exothermic.
From slideplayer.com
Chapter 14 Daily Questions ppt download Is Shaking A Cold Pack Endothermic Or Exothermic These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. In an endothermic reaction, a substance takes heat. An exothermic chemical reaction causes an increase in temperature (of the surroundings). In the course of an endothermic process, the system gains heat from the surroundings. Is Shaking A Cold Pack Endothermic Or Exothermic.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Is Shaking A Cold Pack Endothermic Or Exothermic The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. In an endothermic reaction, a substance takes heat. As for the cold pak, that is an endothermic reaction. These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals,. Is Shaking A Cold Pack Endothermic Or Exothermic.
From slideplayer.com
Endothermic and exothermic reactions ppt download Is Shaking A Cold Pack Endothermic Or Exothermic These cold packs have a strong outer plastic layer that holds a bag of water and a chemical, or mixture of chemicals, that result in an. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. In. Is Shaking A Cold Pack Endothermic Or Exothermic.
From letstalkscience.ca
The Cold Pack A Chilly Example of an Endothermic Reaction Let's Talk Is Shaking A Cold Pack Endothermic Or Exothermic In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. An exothermic chemical reaction causes an increase in temperature (of the surroundings). The changes in energy that occur during. Is Shaking A Cold Pack Endothermic Or Exothermic.
From slideplayer.com
Unit 9H Lesson 5 Endothermic and Exothermic Reactions ppt download Is Shaking A Cold Pack Endothermic Or Exothermic In an endothermic reaction, a substance takes heat. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. As for the cold pak, that is an endothermic reaction. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases.. Is Shaking A Cold Pack Endothermic Or Exothermic.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Is Shaking A Cold Pack Endothermic Or Exothermic An exothermic chemical reaction causes an increase in temperature (of the surroundings). The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Learn about the chemical reactions used to make cold packs and which chemicals to mix to cause endothermic reactions. Exothermic reactions transfer energy to the surroundings and the. Is Shaking A Cold Pack Endothermic Or Exothermic.
From fphoto.photoshelter.com
science chemical reaction endothermic cold pack Fundamental Is Shaking A Cold Pack Endothermic Or Exothermic In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. An exothermic chemical reaction causes an increase in temperature (of the surroundings). In an endothermic reaction, a substance. Is Shaking A Cold Pack Endothermic Or Exothermic.