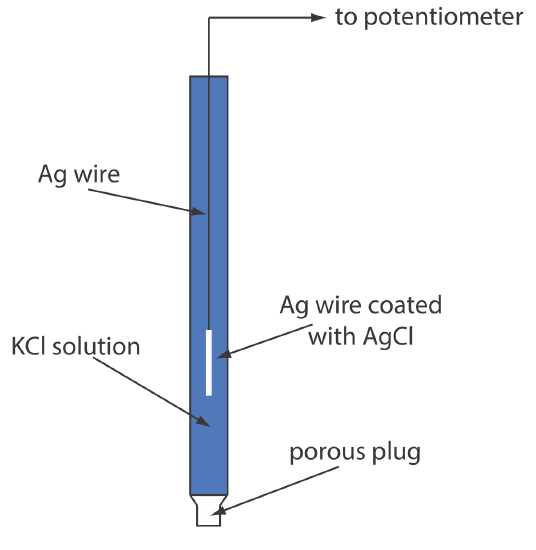
Definition Of Reference Electrodes In Chemistry . Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. In an electrochemical cell, the reference electrode. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. A reference electrode refers to an electrode that has an established electrode potential. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. The indicator electrode possesses some.
from chem.libretexts.org
A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. A reference electrode refers to an electrode that has an established electrode potential. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. The indicator electrode possesses some. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. In an electrochemical cell, the reference electrode. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to.
23.1 Reference Electrodes Chemistry LibreTexts
Definition Of Reference Electrodes In Chemistry Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. A reference electrode refers to an electrode that has an established electrode potential. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. In an electrochemical cell, the reference electrode. The indicator electrode possesses some. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel.
From www.youtube.com
Reference Electrodes YouTube Definition Of Reference Electrodes In Chemistry In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. In an electrochemical cell, the reference electrode. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. A reference electrode refers to an electrode that has an established electrode potential. The indicator electrode possesses some.. Definition Of Reference Electrodes In Chemistry.
From gaskatel.de
Reference electrode Gaskatel Definition Of Reference Electrodes In Chemistry In an electrochemical cell, the reference electrode. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode refers to an electrode that has an established electrode potential. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode scans. Definition Of Reference Electrodes In Chemistry.
From studylib.net
Reference Electrodes Definition Of Reference Electrodes In Chemistry A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. In an electrochemical cell, the reference electrode. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. In this article, we will learn about a primary reference electrode and its type,. Definition Of Reference Electrodes In Chemistry.
From www.mdpi.com
Encyclopedia Free FullText Reference Electrodes Definition Of Reference Electrodes In Chemistry In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. In an electrochemical cell,. Definition Of Reference Electrodes In Chemistry.
From www.mdpi.com
Encyclopedia Free FullText Reference Electrodes Definition Of Reference Electrodes In Chemistry In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A. Definition Of Reference Electrodes In Chemistry.
From www.vedantu.com
Define the reference electrode. Definition Of Reference Electrodes In Chemistry In an electrochemical cell, the reference electrode. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. A reference electrode refers to an electrode that has an established electrode potential. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. The. Definition Of Reference Electrodes In Chemistry.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Definition Of Reference Electrodes In Chemistry In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. A reference electrode refers to an electrode that has an established electrode potential. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during. Definition Of Reference Electrodes In Chemistry.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts Definition Of Reference Electrodes In Chemistry A reference electrode refers to an electrode that has an established electrode potential. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. A reference electrode is a stable. Definition Of Reference Electrodes In Chemistry.
From ppt-online.org
Electrolysis презентация онлайн Definition Of Reference Electrodes In Chemistry In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. A reference electrode refers to an electrode that has an established electrode potential. In an electrochemical cell, the reference electrode. A reference electrode scans the. Definition Of Reference Electrodes In Chemistry.
From chem.libretexts.org
23.1 Reference Electrodes Chemistry LibreTexts Definition Of Reference Electrodes In Chemistry The indicator electrode possesses some. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference. Definition Of Reference Electrodes In Chemistry.
From www.numerade.com
SOLVED Define reference electrode and what it is used for List and Definition Of Reference Electrodes In Chemistry In an electrochemical cell, the reference electrode. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. A reference electrode scans the solution close to the metal surface and measures the potential. Definition Of Reference Electrodes In Chemistry.
From www.slideserve.com
PPT ION SELECTIVE ELECTRODES PowerPoint Presentation ID2144993 Definition Of Reference Electrodes In Chemistry A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. In this article, we will learn. Definition Of Reference Electrodes In Chemistry.
From exoxpbgzu.blob.core.windows.net
Define Electrodes In Chemistry Terms at Monte Cordell blog Definition Of Reference Electrodes In Chemistry The indicator electrode possesses some. In an electrochemical cell, the reference electrode. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. A reference electrode refers to an electrode that has an established electrode potential. A reference electrode is a stable and known electrode potential used as a comparison. Definition Of Reference Electrodes In Chemistry.
From byjus.com
79.What is reference electrode Definition Of Reference Electrodes In Chemistry In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. In an electrochemical cell, the reference electrode. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to.. Definition Of Reference Electrodes In Chemistry.
From mungfali.com
Reference Electrode Diagram Definition Of Reference Electrodes In Chemistry A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. The indicator electrode. Definition Of Reference Electrodes In Chemistry.
From www.slideserve.com
PPT Electrochemical cells PowerPoint Presentation, free download ID Definition Of Reference Electrodes In Chemistry The indicator electrode possesses some. In an electrochemical cell, the reference electrode. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode refers to an electrode that has an established electrode potential. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. A reference. Definition Of Reference Electrodes In Chemistry.
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas Definition Of Reference Electrodes In Chemistry A reference electrode refers to an electrode that has an established electrode potential. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. In an electrochemical cell, the reference electrode. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the. Definition Of Reference Electrodes In Chemistry.
From www.mdpi.com
Encyclopedia Free FullText Reference Electrodes Definition Of Reference Electrodes In Chemistry Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. A reference electrode refers to an electrode. Definition Of Reference Electrodes In Chemistry.
From www.priyamstudycentre.com
Ion Selective Electrode Principle, Types, Application Definition Of Reference Electrodes In Chemistry In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode refers to an electrode that has an established electrode potential. In an electrochemical cell, the reference electrode. A reference electrode scans the solution close. Definition Of Reference Electrodes In Chemistry.
From www.slideserve.com
PPT 146 Cells as chemical probes PowerPoint Presentation, free Definition Of Reference Electrodes In Chemistry A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode refers to an electrode that has an established electrode potential. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. The indicator electrode possesses some. A reference electrode scans. Definition Of Reference Electrodes In Chemistry.
From www.youtube.com
Electrochemistry 6 Type of Electrodes YouTube Definition Of Reference Electrodes In Chemistry Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. A. Definition Of Reference Electrodes In Chemistry.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Definition Of Reference Electrodes In Chemistry A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. The indicator electrode possesses some. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements). Definition Of Reference Electrodes In Chemistry.
From chem.libretexts.org
11.2 Standard Electrode Potentials Chemistry LibreTexts Definition Of Reference Electrodes In Chemistry Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. A reference electrode refers to an. Definition Of Reference Electrodes In Chemistry.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Definition Of Reference Electrodes In Chemistry In an electrochemical cell, the reference electrode. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. The indicator electrode possesses some. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode is a stable and known electrode potential. Definition Of Reference Electrodes In Chemistry.
From ceias.nau.edu
Potentiometry Definition Of Reference Electrodes In Chemistry The indicator electrode possesses some. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical. Definition Of Reference Electrodes In Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Definition Of Reference Electrodes In Chemistry The indicator electrode possesses some. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or. Definition Of Reference Electrodes In Chemistry.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Definition Of Reference Electrodes In Chemistry Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. The indicator electrode possesses some. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference. Definition Of Reference Electrodes In Chemistry.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry Definition Of Reference Electrodes In Chemistry A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. A reference electrode refers to an electrode that has. Definition Of Reference Electrodes In Chemistry.
From www.slideserve.com
PPT Electroanalysis PowerPoint Presentation, free download ID6733376 Definition Of Reference Electrodes In Chemistry The indicator electrode possesses some. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and. Definition Of Reference Electrodes In Chemistry.
From mungfali.com
Types Of Electrodes Definition Of Reference Electrodes In Chemistry In an electrochemical cell, the reference electrode. The indicator electrode possesses some. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode refers to an electrode that has an established electrode. Definition Of Reference Electrodes In Chemistry.
From studylib.net
Electrodes and Potentiometry Introduction 1.) Potentiometry Definition Of Reference Electrodes In Chemistry In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. In an electrochemical cell, the reference electrode. A reference electrode scans the solution close to the metal surface and measures the potential referred to another reference electrode placed. In potentiometry, those two electrodes are generally called the indicator electrode and. Definition Of Reference Electrodes In Chemistry.
From www.toppr.com
Describe the construction and working of calomel electrode. Definition Of Reference Electrodes In Chemistry Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode refers to an electrode that has an established electrode potential. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. A reference electrode scans the solution close to the. Definition Of Reference Electrodes In Chemistry.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Definition Of Reference Electrodes In Chemistry In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. A reference electrode refers to an electrode that has an established electrode potential. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. In an electrochemical cell, the reference electrode. The indicator electrode possesses some. A reference. Definition Of Reference Electrodes In Chemistry.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Definition Of Reference Electrodes In Chemistry In an electrochemical cell, the reference electrode. The indicator electrode possesses some. In this article, we will learn about a primary reference electrode and its type, i.e., standard hydrogen electrode and calomel. Reference electrodes are necessary to control the potential of a working electrode (e.g., during voltammetric measurements) or to. A reference electrode refers to an electrode that has an. Definition Of Reference Electrodes In Chemistry.
From askfilo.com
29. Define reference electrode. Write the functions of salt bridge. Draw Definition Of Reference Electrodes In Chemistry In potentiometry, those two electrodes are generally called the indicator electrode and the reference electrode. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. A reference electrode refers to an electrode that has an established electrode potential. In an electrochemical cell, the reference electrode. The indicator electrode possesses some. Reference electrodes. Definition Of Reference Electrodes In Chemistry.