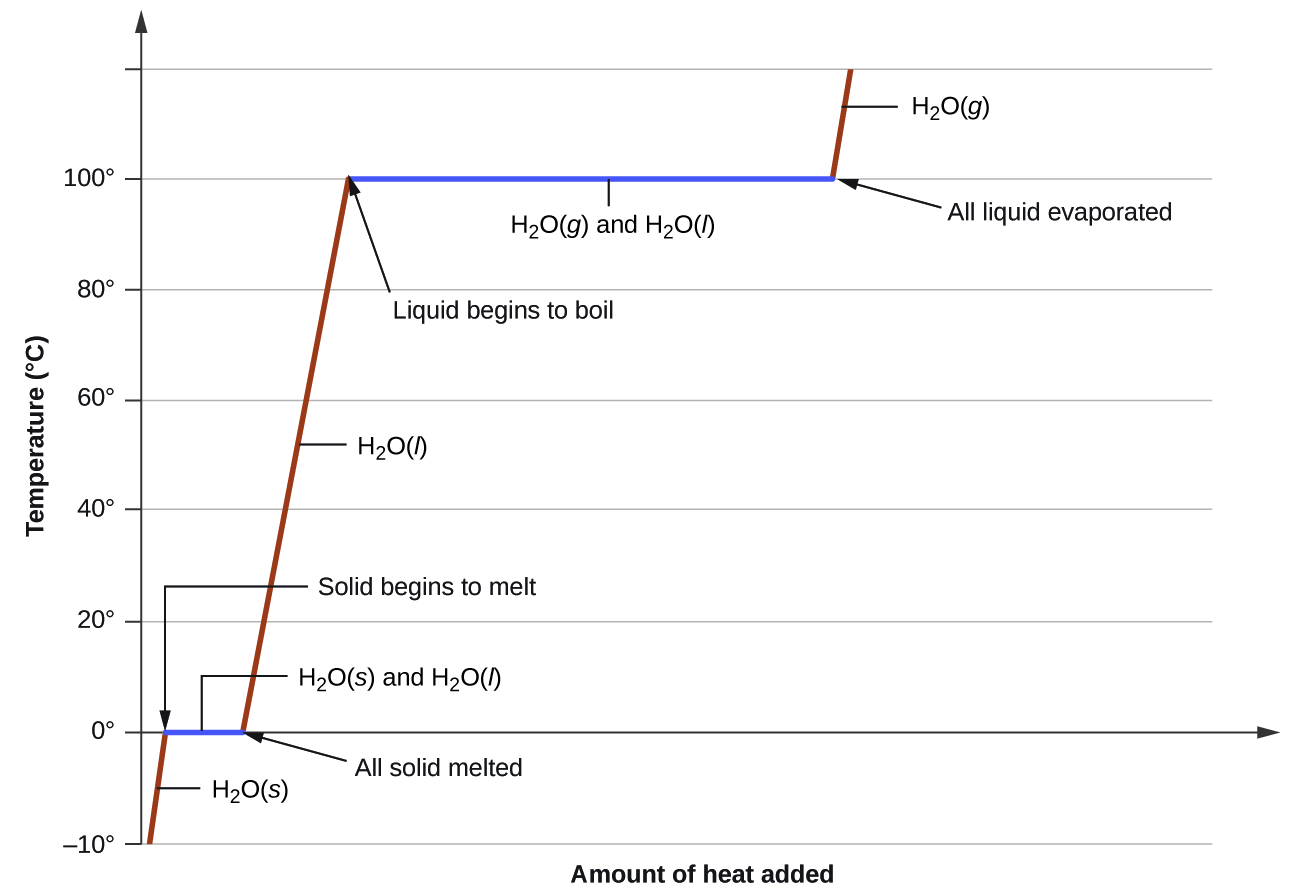
Kent Chemistry Heating Curve . The melting and boiling points of the substance can be determined by the. N37 heating and cooling curves. The sample is initially ice at 1 atm. I can use heating and cooling curves to help calculate the energy changes. The change of state behavior of all substances can be represented with a heating curve of this type. Cooling curves are the opposite. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. Heating curves show how the temperature changes as a substance is heated up. The line between the liquid and gas phases is a curve of all the boiling points of the substance. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water.
from chem.libretexts.org
9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. The melting and boiling points of the substance can be determined by the. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. I can use heating and cooling curves to help calculate the energy changes. Heating curves show how the temperature changes as a substance is heated up. The change of state behavior of all substances can be represented with a heating curve of this type. Cooling curves are the opposite. N37 heating and cooling curves. The sample is initially ice at 1 atm. The line between the liquid and gas phases is a curve of all the boiling points of the substance.
3.7.0 Heating Curves and Phase Changes Chemistry LibreTexts
Kent Chemistry Heating Curve The change of state behavior of all substances can be represented with a heating curve of this type. The change of state behavior of all substances can be represented with a heating curve of this type. I can use heating and cooling curves to help calculate the energy changes. N37 heating and cooling curves. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The sample is initially ice at 1 atm. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. The line between the liquid and gas phases is a curve of all the boiling points of the substance. Heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. The melting and boiling points of the substance can be determined by the.
From www.youtube.com
Heating and Cooling Curves IGCSE/ O level Chemistry / lec6 Chapter1 Kent Chemistry Heating Curve Heating curves show how the temperature changes as a substance is heated up. The melting and boiling points of the substance can be determined by the. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The line between the liquid and gas phases is a curve of all the. Kent Chemistry Heating Curve.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Kent Chemistry Heating Curve The sample is initially ice at 1 atm. I can use heating and cooling curves to help calculate the energy changes. The line between the liquid and gas phases is a curve of all the boiling points of the substance. Cooling curves are the opposite. The change of state behavior of all substances can be represented with a heating curve. Kent Chemistry Heating Curve.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Kent Chemistry Heating Curve The change of state behavior of all substances can be represented with a heating curve of this type. The melting and boiling points of the substance can be determined by the. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. N37 heating and cooling curves. Cooling curves are the. Kent Chemistry Heating Curve.
From evulpo.com
Heating and cooling curves Science Explanation & Exercises evulpo Kent Chemistry Heating Curve The line between the liquid and gas phases is a curve of all the boiling points of the substance. Cooling curves are the opposite. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. N37 heating and cooling curves. Figure \(\pageindex{3}\) shows. Kent Chemistry Heating Curve.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Kent Chemistry Heating Curve Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The melting and boiling points of the substance can be determined by the. The line between the liquid and gas phases is a curve of all the boiling points of the substance. I can use heating and cooling curves to. Kent Chemistry Heating Curve.
From chem.libretexts.org
8.1 Heating Curves and Phase Changes Chemistry LibreTexts Kent Chemistry Heating Curve N37 heating and cooling curves. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The line between the liquid and gas phases is a curve of all the boiling points of the substance. The sample is initially ice at 1 atm. The melting and boiling points of the substance. Kent Chemistry Heating Curve.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Kent Chemistry Heating Curve The melting and boiling points of the substance can be determined by the. Cooling curves are the opposite. Heating curves show how the temperature changes as a substance is heated up. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. 9.the graph below shows the heating curve of 1.0. Kent Chemistry Heating Curve.
From studyschoolburman.z21.web.core.windows.net
Heating And Cooling Curves Explained Kent Chemistry Heating Curve The melting and boiling points of the substance can be determined by the. The sample is initially ice at 1 atm. The line between the liquid and gas phases is a curve of all the boiling points of the substance. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant. Kent Chemistry Heating Curve.
From byjus.com
Explain the cooling curve in crystalline and amorphous solids. Kent Chemistry Heating Curve 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. Heating curves show how the temperature changes as a substance is heated up. The change of state behavior of all substances can be represented with a heating curve of this type. N37. Kent Chemistry Heating Curve.
From ar.inspiredpencil.com
Heating Curves In Chemistry Kent Chemistry Heating Curve The line between the liquid and gas phases is a curve of all the boiling points of the substance. I can use heating and cooling curves to help calculate the energy changes. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting.. Kent Chemistry Heating Curve.
From quizizz.com
Heating Curve Graphs Chemistry Quizizz Kent Chemistry Heating Curve Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The line between the liquid and gas phases is a curve of all the boiling points of the substance. Cooling curves are the opposite. N37 heating and cooling curves. Heating curves show how the temperature changes as a substance is. Kent Chemistry Heating Curve.
From preparatorychemistry.com
Heating Curve Kent Chemistry Heating Curve Heating curves show how the temperature changes as a substance is heated up. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. I can use heating and cooling curves to help calculate the energy changes. The change of state behavior of. Kent Chemistry Heating Curve.
From quizzschoolhernandez.z19.web.core.windows.net
Heating Curve Of Water Explained Kent Chemistry Heating Curve Cooling curves are the opposite. The line between the liquid and gas phases is a curve of all the boiling points of the substance. N37 heating and cooling curves. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. The change of. Kent Chemistry Heating Curve.
From www.savemyexams.com
Limitations of the Particle Model OCR Gateway GCSE Chemistry Revision Kent Chemistry Heating Curve N37 heating and cooling curves. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. I can use heating and cooling curves. Kent Chemistry Heating Curve.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Kent Chemistry Heating Curve I can use heating and cooling curves to help calculate the energy changes. The melting and boiling points of the substance can be determined by the. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. The change of state behavior of. Kent Chemistry Heating Curve.
From www.albert.io
Heating Curve and Specific Heat Chemistry Practice Albert Kent Chemistry Heating Curve The line between the liquid and gas phases is a curve of all the boiling points of the substance. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The change of state behavior of all substances can be represented with a heating curve of this type. Cooling curves are. Kent Chemistry Heating Curve.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Kent Chemistry Heating Curve I can use heating and cooling curves to help calculate the energy changes. The line between the liquid and gas phases is a curve of all the boiling points of the substance. Heating curves show how the temperature changes as a substance is heated up. 9.the graph below shows the heating curve of 1.0 gram of a solid as it. Kent Chemistry Heating Curve.
From answerzoneschuster.z21.web.core.windows.net
Heat And Cooling Curve Kent Chemistry Heating Curve The change of state behavior of all substances can be represented with a heating curve of this type. Heating curves show how the temperature changes as a substance is heated up. I can use heating and cooling curves to help calculate the energy changes. N37 heating and cooling curves. 9.the graph below shows the heating curve of 1.0 gram of. Kent Chemistry Heating Curve.
From www.showme.com
Heating Curves Science, Chemistry ShowMe Kent Chemistry Heating Curve The line between the liquid and gas phases is a curve of all the boiling points of the substance. The melting and boiling points of the substance can be determined by the. I can use heating and cooling curves to help calculate the energy changes. Heating curves show how the temperature changes as a substance is heated up. 9.the graph. Kent Chemistry Heating Curve.
From www.youtube.com
How to Read a Heating Curve YouTube Kent Chemistry Heating Curve The change of state behavior of all substances can be represented with a heating curve of this type. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. The line between the liquid and gas phases is a curve of all the. Kent Chemistry Heating Curve.
From www.youtube.com
IGCSE Chemistry Heating curve YouTube Kent Chemistry Heating Curve 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. The line between the liquid and gas phases is a curve of all the boiling points of the substance. The melting and boiling points of the substance can be determined by the.. Kent Chemistry Heating Curve.
From schematiclistmorvant.z13.web.core.windows.net
Heating Curve Diagram Kent Chemistry Heating Curve 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. The sample is initially ice at 1 atm. The change of state behavior of all substances can be represented with a heating curve of this type. I can use heating and cooling. Kent Chemistry Heating Curve.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Kent Chemistry Heating Curve The line between the liquid and gas phases is a curve of all the boiling points of the substance. The sample is initially ice at 1 atm. I can use heating and cooling curves to help calculate the energy changes. The melting and boiling points of the substance can be determined by the. The change of state behavior of all. Kent Chemistry Heating Curve.
From mavink.com
What Is A Heating Curve Kent Chemistry Heating Curve Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The sample is initially ice at 1 atm. The line between the liquid and gas phases is a curve of all the boiling points of the substance. Cooling curves are the opposite. I can use heating and cooling curves to. Kent Chemistry Heating Curve.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book Kent Chemistry Heating Curve The melting and boiling points of the substance can be determined by the. The sample is initially ice at 1 atm. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. I can use heating and cooling curves to help calculate the. Kent Chemistry Heating Curve.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Kent Chemistry Heating Curve N37 heating and cooling curves. Heating curves show how the temperature changes as a substance is heated up. I can use heating and cooling curves to help calculate the energy changes. The sample is initially ice at 1 atm. The melting and boiling points of the substance can be determined by the. Figure \(\pageindex{3}\) shows a heating curve, a plot. Kent Chemistry Heating Curve.
From lessonlibsertularia.z22.web.core.windows.net
Heating And Cooling Curves Explained Kent Chemistry Heating Curve Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. N37 heating and cooling curves. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. Cooling curves are the opposite. The change. Kent Chemistry Heating Curve.
From chem.libretexts.org
3.7.0 Heating Curves and Phase Changes Chemistry LibreTexts Kent Chemistry Heating Curve N37 heating and cooling curves. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. I can use heating and cooling curves to help calculate the energy changes. The line between the liquid and gas phases is a curve of all the. Kent Chemistry Heating Curve.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic Kent Chemistry Heating Curve I can use heating and cooling curves to help calculate the energy changes. The melting and boiling points of the substance can be determined by the. The line between the liquid and gas phases is a curve of all the boiling points of the substance. Cooling curves are the opposite. 9.the graph below shows the heating curve of 1.0 gram. Kent Chemistry Heating Curve.
From studyschoolburman.z21.web.core.windows.net
Heating And Cooling Curves Explained Kent Chemistry Heating Curve Cooling curves are the opposite. The line between the liquid and gas phases is a curve of all the boiling points of the substance. The sample is initially ice at 1 atm. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. N37 heating and cooling curves. The melting and. Kent Chemistry Heating Curve.
From quizlet.com
Advanced Chemistry Heating Curve Diagram Quizlet Kent Chemistry Heating Curve Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Cooling curves are the opposite. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. The change of state behavior of all. Kent Chemistry Heating Curve.
From hrsbstaff.ednet.ns.ca
Science 10 Kent Chemistry Heating Curve 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. The melting and boiling points of the substance can be determined by the. Cooling curves are the opposite. N37 heating and cooling curves. I can use heating and cooling curves to help. Kent Chemistry Heating Curve.
From evulpo.com
Heating and cooling curves Science Explanation & Exercises evulpo Kent Chemistry Heating Curve The sample is initially ice at 1 atm. 9.the graph below shows the heating curve of 1.0 gram of a solid as it is heated at a constant rate, starting at a temperature below its melting. N37 heating and cooling curves. The change of state behavior of all substances can be represented with a heating curve of this type. The. Kent Chemistry Heating Curve.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Kent Chemistry Heating Curve The change of state behavior of all substances can be represented with a heating curve of this type. The sample is initially ice at 1 atm. Heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. The line between the liquid and gas phases is a curve of all the boiling points. Kent Chemistry Heating Curve.
From www.smartexamresources.com
IGCSE Chemistry Notes Solids, Liquids And Gases Smart Exam Resources Kent Chemistry Heating Curve Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. I can use heating and cooling curves to help calculate the energy changes. The melting and boiling points of the substance can be determined by the. The change of state behavior of all substances can be represented with a heating. Kent Chemistry Heating Curve.