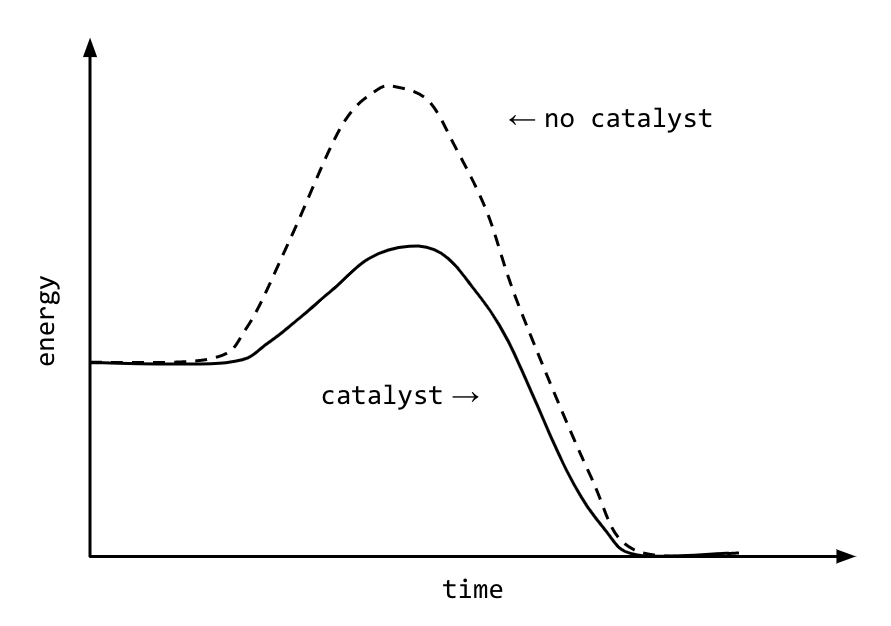
Catalyst Chemical Reaction Energy . Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. This process is called catalysis. List examples of catalysis in natural. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalysts participate in a chemical reaction and increase its rate. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. It is not consumed by the process. In chemistry and biology, a catalyst is a substance the increases. Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts. A catalyst is a chemical substance. A catalyst lowers the activation energy of a reaction, increasing its rate.
from nesslabs.com
A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. It is not consumed by the process. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is a chemical substance. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. List examples of catalysis in natural. Catalysts participate in a chemical reaction and increase its rate.
Activation energy the chemistry of getting started Ness Labs
Catalyst Chemical Reaction Energy Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. It is not consumed by the process. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts participate in a chemical reaction and increase its rate. In chemistry and biology, a catalyst is a substance the increases. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a chemical substance. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. A catalyst lowers the activation energy of a reaction, increasing its rate. This process is called catalysis. List examples of catalysis in natural. Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalyst Chemical Reaction Energy A catalyst is a chemical substance. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts. A catalyst. Catalyst Chemical Reaction Energy.
From www.researchgate.net
Chemical Reaction Energy Profile Catalysts reduce the activation energy Catalyst Chemical Reaction Energy A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. In chemistry and biology, a catalyst is a substance the increases. It is not consumed by the process. This process is called catalysis. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts participate in a chemical reaction and. Catalyst Chemical Reaction Energy.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.9.3 MaxwellBoltzmann Distributions翰林国际教育 Catalyst Chemical Reaction Energy List examples of catalysis in natural. This process is called catalysis. A catalyst is a chemical substance. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. Learn all. Catalyst Chemical Reaction Energy.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Catalyst Chemical Reaction Energy A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. In chemistry and biology, a catalyst is a substance the increases. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. This process is called catalysis. Explain. Catalyst Chemical Reaction Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalyst Chemical Reaction Energy A catalyst is a chemical substance. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. In chemistry and biology, a catalyst is a substance the increases. Catalysts participate in. Catalyst Chemical Reaction Energy.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Chemical Reaction Energy A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst lowers the activation energy of a reaction, increasing its rate. It is not consumed by the process. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction. Catalyst Chemical Reaction Energy.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Chemical Reaction Energy This process is called catalysis. It is not consumed by the process. Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts. A catalyst lowers the activation energy of a reaction, increasing its rate. A catalyst is a chemical substance. In chemistry and biology, a catalyst is a substance the increases. Catalysts allow. Catalyst Chemical Reaction Energy.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Chemical Reaction Energy A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst lowers the activation energy of a reaction, increasing its rate. List examples of catalysis in natural. A catalyst is a chemical substance. Catalysis is the process that alters the rate of a chemical. Catalyst Chemical Reaction Energy.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Chemical Reaction Energy They do not appear in the reaction’s net equation and are not consumed during the reaction. It is not consumed by the process. A catalyst lowers the activation energy of a reaction, increasing its rate. In chemistry and biology, a catalyst is a substance the increases. A catalyst is a substance that increases the rate of a chemical reaction by. Catalyst Chemical Reaction Energy.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Chemical Reaction Energy It is not consumed by the process. List examples of catalysis in natural. Catalysts participate in a chemical reaction and increase its rate. Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required. Catalyst Chemical Reaction Energy.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Catalyst Chemical Reaction Energy Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. It is not consumed by the process. List examples of catalysis in natural. A catalyst is a chemical substance. A catalyst is a chemical substance that affects the rate of a. Catalyst Chemical Reaction Energy.
From 2012books.lardbucket.org
Catalysis Catalyst Chemical Reaction Energy This process is called catalysis. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst lowers the activation energy of a reaction, increasing its rate. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. It is not consumed by the process. A catalyst is. Catalyst Chemical Reaction Energy.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Chemical Reaction Energy List examples of catalysis in natural. In chemistry and biology, a catalyst is a substance the increases. A catalyst is a chemical substance. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts participate in a chemical reaction and increase its rate. Learn all. Catalyst Chemical Reaction Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Catalyst Chemical Reaction Energy A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Learn all about catalysts of chemical reactions, what is. Catalyst Chemical Reaction Energy.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalyst Chemical Reaction Energy A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. It is not consumed by the process. A. Catalyst Chemical Reaction Energy.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalyst Chemical Reaction Energy A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. It is not consumed by the process. Catalysis is the process that alters the rate of a. Catalyst Chemical Reaction Energy.
From schoolbag.info
A catalyst speeds up a reaction by providing the reactants with an Catalyst Chemical Reaction Energy A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. They do not appear in the reaction’s net equation and are not consumed during the reaction. This process is called catalysis. It is not consumed by the process. Learn all about catalysts of chemical reactions, what is activation energy, and different types. Catalyst Chemical Reaction Energy.
From www.nagwa.com
Question Video Determining How a Catalyst Affects the Energy Profile Catalyst Chemical Reaction Energy Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. List examples of catalysis in natural. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. This process is called catalysis. In chemistry and biology, a catalyst is a substance the increases. Catalysis is the process. Catalyst Chemical Reaction Energy.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst Chemical Reaction Energy Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst lowers the activation energy of a reaction, increasing its rate. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Learn all about catalysts of chemical reactions, what is activation energy,. Catalyst Chemical Reaction Energy.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Catalyst Chemical Reaction Energy Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts. A catalyst is a chemical substance. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysis is the process that alters the rate of a chemical reaction. Catalyst Chemical Reaction Energy.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Chemical Reaction Energy Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This process is called catalysis. A catalyst lowers the activation energy of a reaction, increasing its rate. Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts. Catalysts participate in a chemical reaction and increase. Catalyst Chemical Reaction Energy.
From www.slideserve.com
PPT KEY CONCEPT Enzymes are catalysts for chemical reactions in Catalyst Chemical Reaction Energy Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. It is not consumed by the process.. Catalyst Chemical Reaction Energy.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Chemical Reaction Energy Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts. Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. List examples of catalysis in natural. It is not consumed by the process. A catalyst is not. Catalyst Chemical Reaction Energy.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalyst Chemical Reaction Energy Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. In chemistry and biology, a catalyst is a substance the increases. This process is called catalysis. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A. Catalyst Chemical Reaction Energy.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Catalyst Chemical Reaction Energy This process is called catalysis. They do not appear in the reaction’s net equation and are not consumed during the reaction. Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts. It is not consumed by the process. A catalyst is a substance that increases the rate of a chemical reaction by lowering. Catalyst Chemical Reaction Energy.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyst Chemical Reaction Energy A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. This process is called catalysis. In chemistry and biology, a catalyst is a substance the increases. It is not consumed by the process. Catalysts allow a reaction to proceed via a pathway that has a. Catalyst Chemical Reaction Energy.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Chemical Reaction Energy List examples of catalysis in natural. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is not consumed by the reaction and it may. Catalyst Chemical Reaction Energy.
From www.catalystseurope.org
What are catalysts? Catalyst Chemical Reaction Energy Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts participate in a chemical reaction and increase its rate. A catalyst is a chemical substance. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalysts allow a reaction to proceed via a pathway that has. Catalyst Chemical Reaction Energy.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst Chemical Reaction Energy A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. It is not consumed by the process. A catalyst is a chemical. Catalyst Chemical Reaction Energy.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Catalyst Chemical Reaction Energy A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical substance. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts allow a. Catalyst Chemical Reaction Energy.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyst Chemical Reaction Energy A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts. Catalysts participate in a chemical reaction and increase its rate. A catalyst is a substance that increases the rate of a chemical reaction by lowering. Catalyst Chemical Reaction Energy.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Chemical Reaction Energy A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. A catalyst lowers the activation energy of a reaction, increasing its rate. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a. Catalyst Chemical Reaction Energy.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst Chemical Reaction Energy This process is called catalysis. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation. Catalyst Chemical Reaction Energy.
From vdocuments.mx
Catalysts Catalystlowers activation energy of a chemical reaction and Catalyst Chemical Reaction Energy In chemistry and biology, a catalyst is a substance the increases. It is not consumed by the process. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts. They do not appear in the. Catalyst Chemical Reaction Energy.
From www.pnnl.gov
Catalysis PNNL Catalyst Chemical Reaction Energy A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Learn all about catalysts of chemical reactions, what is activation energy, and different types of common catalysts. A catalyst lowers the activation energy of a reaction, increasing its rate. Explain the function of a catalyst in terms of reaction mechanisms and potential. Catalyst Chemical Reaction Energy.